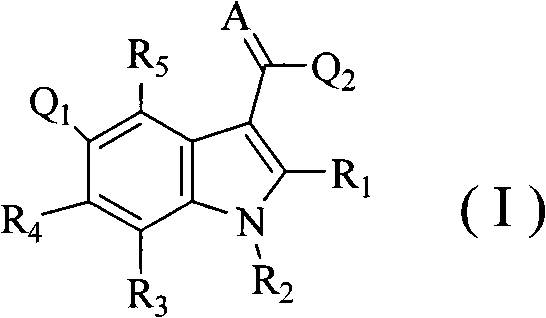
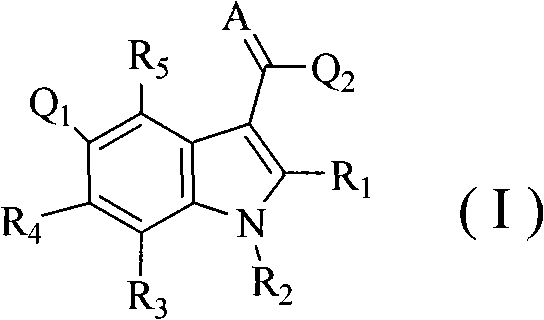
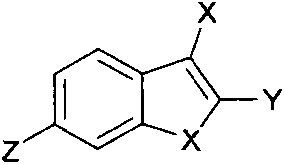
Substituent indole compound and application thereof
A technology of compounds, indoles, applied in applications, organic chemistry, biocides, etc., which can solve problems such as structural differences
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0133] Example 1: Preparation of compound 35
[0134] (1) Synthesis of intermediate (V-A)
[0135]
[0136] Add 7.0 g of p-fluoroaniline to 20 ml of oxalyl chloride, heat at 165°C for 30 minutes, distill the excess oxalyl chloride to obtain a light yellow oil, let stand to cool, add a small amount of methanol, solids precipitate, filter, and methanol wash The target product is 8.3 g, yield: 80%.
[0137] (2) Synthesis of intermediate (IV-A)
[0138]
[0139] Heat 2.2 g of 5-fluoroindole-2,3-dione (VA) with 4 ml of 85% hydrazine hydrate in ethanol solution for 4 hours at reflux, add 2.8 g of sodium hydroxide, and continue the reaction 4 After hours, it was extracted with ethyl acetate and dried over anhydrous magnesium sulfate. Under reduced pressure distillation, 1.5 g of yellow solid was obtained, the melting point was 143-145°C, and the yield was 74.6%.
[0140] (3) Synthesis of compound 35
[0141]
[0142] Under the ice-salt bath, slowly drop 1.2 g of phosphorus oxychloride into t...
example 2
[0144] Example 2: Preparation of compound 41
[0145]
[0146] Add 1 g of 2-chloro-5-fluoroindole-3-carbaldehyde (Compound 35) into the reaction flask, add 0.4 g of hydroxylamine hydrochloride, 0.2 g of sodium hydroxide, 40 ml of ethanol, and heat at 80°C with stirring and reflux for reaction. After the reaction was completed, the reaction solution was poured into water and extracted with ethyl acetate to obtain 0.8 g of yellow solid, yield: 64.7%.
example 3
[0147] Example 3: Preparation of compound 46
[0148]
[0149] At room temperature, 0.2 g of compound 41 was added to 0.3 g of p-toluic acid in methylene chloride solution, and 0.5 g of triethylamine was added. React at room temperature for 2 hours. Extract with dichloromethane, wash with saturated sodium bicarbonate, and dry with anhydrous magnesium sulfate. Concentrated to obtain 0.11 g of red solid, melting point 150-154°C, yield: 35.4%. The NMR data are as follows:
[0150] 1 H-NMR (300MHz, internal standard TMS, solvent CDCl 3 )δ(ppm): 8.68(s, 1H, NH), 7.91(s, 1H, CH), 7.85-7.28(m, 7H, Ar-H), 2.43(s, 3H, CH) 3 ).
[0151] The physical and chemical properties and nuclear magnetic data of some compounds ( 1 HNMR, 300MHz, internal standard TMS, solvent CDCl 3 )as follows:
[0152] Compound 1: Melting point is 266-268°C. δ ppm 9.97 (s, 1H, CHO), 8.02 (d, 1H, indole-4-H), 7.46 (dd, 1H, indole-7-H), 7.32 (dd, 1H, indole-6-H).
[0153] Compound 22: Melting point is 260-262°C. δ ppm ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com