Preparation method of stannous methanesulfonate
A technology of stannous methanesulfonate and methanesulfonic acid, which is applied in the field of stannous methanesulfonate preparation, can solve the problems of long reaction time, high reaction temperature, and many by-products, and achieve fast reaction speed and post-processing The effect of simplicity and short production cycles
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
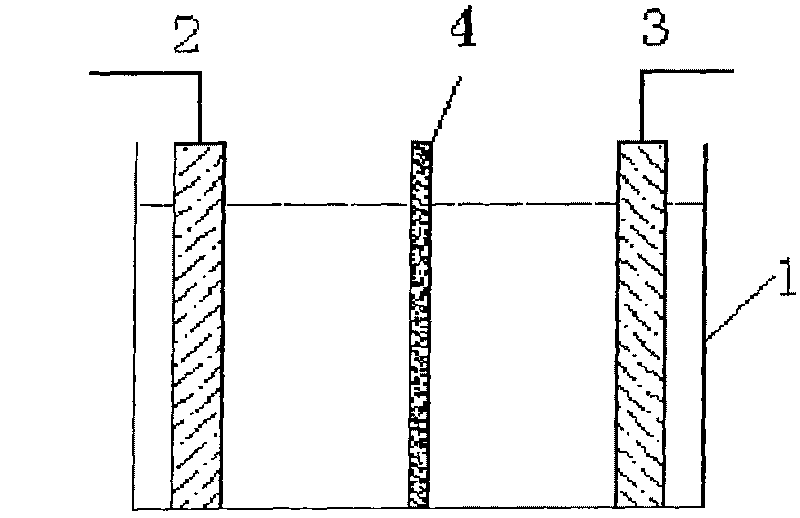
[0016] Embodiment 1: Referring to the accompanying drawings, the method for preparing stannous methanesulfonate by electrolysis method, in the diaphragm type polypropylene outer frame electrolytic cell 1, there are high-purity tin plate anode 2 and cathode 3 with a purity of 99.9wt% oppositely arranged, and the area is They are 1dm2 respectively, the two electrodes are separated by a secondary amine type anion membrane 4, and the distance between the anode and the cathode and the ion membrane is 3cm, respectively constituting the anode chamber and the cathode chamber. Add 150.0 g of a 70 wt% methanesulfonic acid aqueous solution and 50.0 g of water to the anode chamber, and also add 150.0 g of a 70 wt% methanesulfonic acid aqueous solution and 50.0 g of water to the cathode chamber to conduct electrolysis at a constant current of 10A. The anode tin electrode gradually decreases, and the cathode evolves hydrogen. After electrolysis for a period of time, take a small amount of a...
Embodiment 2-4
[0017] Embodiment 2-4: select different shapes of tin electrodes according to table 1, wherein in the particle tin and powder tin electrolytic device, the plate graphite current collector is added, other experimental devices and process parameters are the same as in embodiment 1, and the electrolysis results are as shown in table 1. Show.
[0018] Table 1 Test comparison table of different forms of tin electrodes
[0019] Example
[0020] The results showed that there was no significant difference in electrolysis between electrodes of various shapes.
Embodiment 5-10
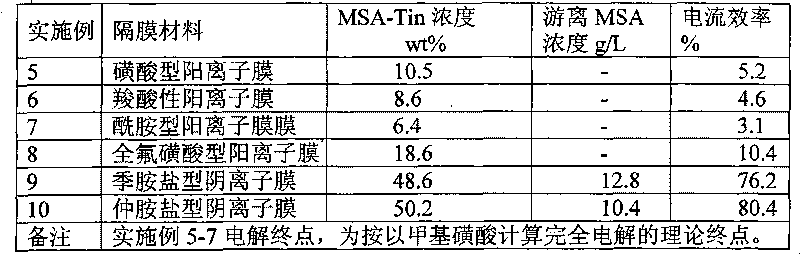
[0021] Example 5-10: According to Table 2, different ion exchange membranes were selected as diaphragm materials, other experimental devices and process parameters were the same as those in Example 1, and the electrolysis results were shown in Table 2.
[0022] Table 2 Test comparison table of different ion exchange membranes
[0023]
[0024] The results show that both anionic membranes and cationic membranes can be electrolyzed, but anionic membranes have better effects than cationic membranes. high, high current efficiency.
[0025] Examples 11-16: Electrolysis was performed with different current densities according to Table 3, other experimental devices and process parameters were the same as those of Example 1, and the electrolysis results were shown in Table 3.
[0026] Table 3 Comparison table of different current density tests
[0027]
[0028] The results show that the effects of various current densities are roughly similar. Obviously, if the current ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com