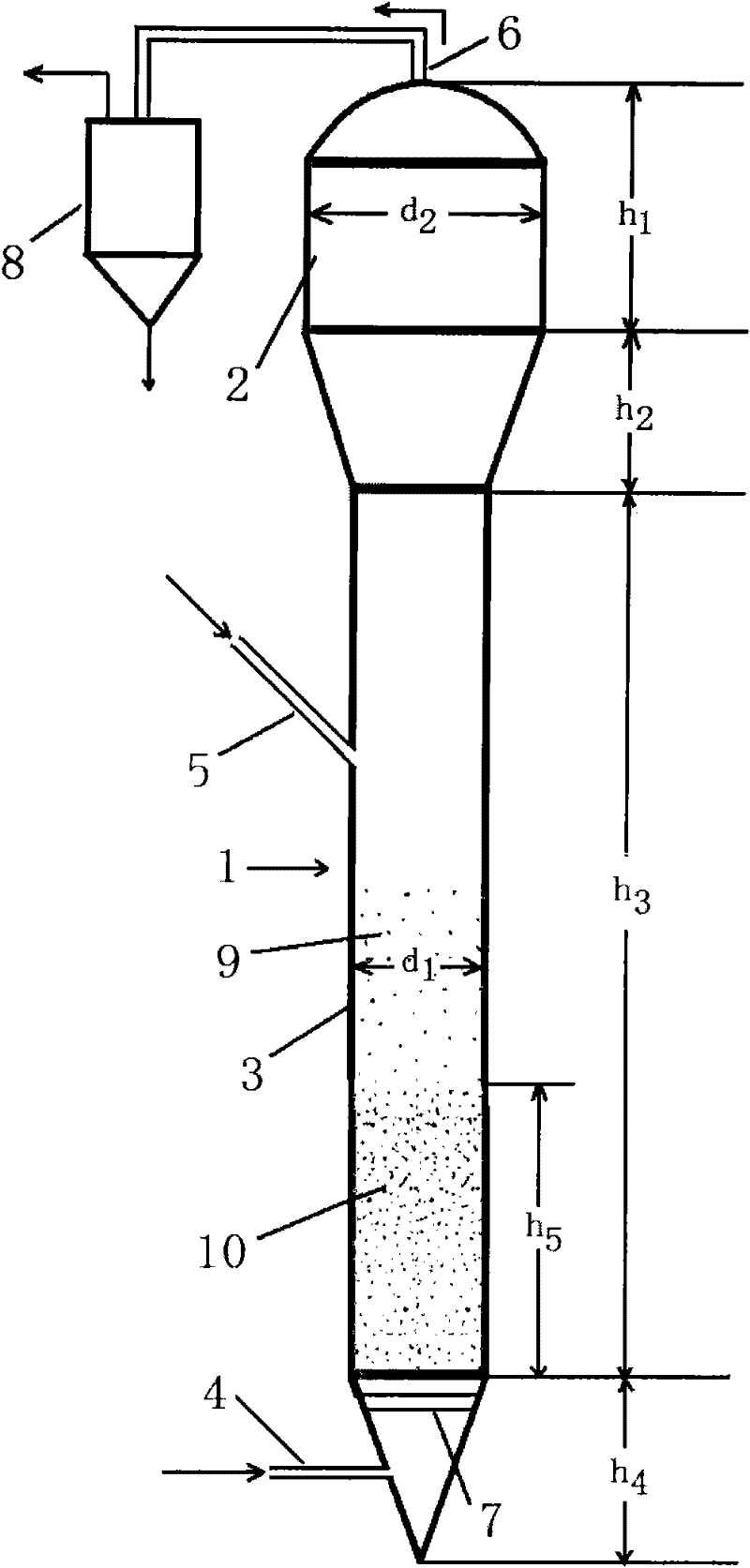
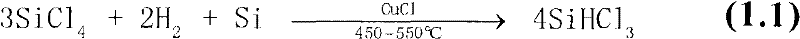Method for preparing trichlorosilane
A technology for the manufacture of trichlorosilane, applied in the direction of halosilane, halide silicon compounds, etc., can solve the problems of difficult production operation, high production cost, high raw material purity requirements, high reaction temperature, etc., and achieve improved single-pass conversion rate, The production operation is less difficult and the effect of lowering the reaction temperature
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0106] The gas mixture consists of hydrogen and silicon tetrachloride in a molar ratio of 2.0:1.
[0107] CuCl is used as the main catalyst and NaCl is used as the co-catalyst, and relative to 100 parts by weight of silicon powder, the amount of CuCl is 1.5 parts by weight, and the amount of NaCl is 0.2 parts by weight.
[0108] The reaction pressure is 2.0MPa, the reaction temperature is 500°C, and the residence time is 26s.
[0109] After the reaction was carried out for 10 hours, the gas product was analyzed, and it was found that the molar content of trichlorosilane in the gas product was 24.7%, and the molar content of silicon tetrachloride was 74.6%. It can be known by calculation that the single pass conversion rate of silicon tetrachloride was 19.89%.
Embodiment 2
[0116] The gas mixture consists of hydrogen and silicon tetrachloride in a molar ratio of 2.0:1.
[0117] CuCl is used as the main catalyst and NaCl is used as the co-catalyst, and relative to 100 parts by weight of silicon powder, the amount of CuCl used is 1.5 parts by weight, and the amount of NaCl used is 0.75 parts by weight.
[0118] The reaction pressure is 2.2MPa, the reaction temperature is 510°C, and the residence time is 24s.
[0119] After the reaction was carried out for 10 hours, the gas product was analyzed, and it was found that the molar content of trichlorosilane in the gas product was 30.6%, and the molar content of silicon tetrachloride was 68.5%. It can be known by calculation that the single pass conversion rate of silicon tetrachloride is 25.10%.
Embodiment 3
[0121] The gas mixture consists of hydrogen, silicon tetrachloride and hydrogen chloride in a molar ratio of 14:7:1.
[0122] NiCl 2 As the main catalyst, KCl is used as the cocatalyst, and relative to 100 parts by weight of silicon powder, the NiCl 2 The consumption of KCl is 1.8 parts by weight, and the consumption of KCl is 0.5 parts by weight.
[0123] The reaction pressure is 2.0MPa, the reaction temperature is 500°C, and the residence time is 26s.
[0124] After the reaction was carried out for 10 hours, the gas product was analyzed, and it was found that the molar content of trichlorosilane in the gas product was 34.7%, and the molar content of silicon tetrachloride was 64.2%. It can be known by calculation that the single pass conversion rate of silicon tetrachloride was 28.84%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com