Compound glycyrrhizin injection preparation
A technology of glycyrrhizin injection and monoammonium glycyrrhizinate, which is applied in pharmaceutical formulations, medical preparations containing active ingredients, organic active ingredients, etc., can solve the problem of inability to obtain sufficient stability, potential safety hazards, and effective ingredients in injection color. , pH changes, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Effect of Addition of Basic Amino Acids on the Solubility of Glycyrrhizin (Monoammonium Glycyrrhizinate)
[0047] Prescription 1 Prescription 2 Prescription 3
[0048] Monoammonium Glycyrrhizinate 5.3g 5.3g 5.3g
[0049] Cysteine Hydrochloride 2g 2g 2g
[0050] Glycine 40g 40g 40g
[0051] L-Arginine - 3.5g -
[0052] L-Lysine - - 2.8g
[0053]Dissolving step: Weigh each recipe composition, put it into a Erlenmeyer flask, heat and stir in a water bath at 40±2°C for 30 minutes, let it cool at room temperature, filter, transfer the filtrate to a 1000ml volumetric flask, distill the volume to the mark with distilled water, and set aside , to determine the content of monoammonium glycyrrhizinate.
[0054] test methods:
[0055] Chromatographic conditions and system suitability test used octadecylsilane bonded silica gel as filler; acetonitrile-0.01mol / L phosphoric acid solution (38:62) as mobile phase; detection wavelength was 257nm. The theoretical...
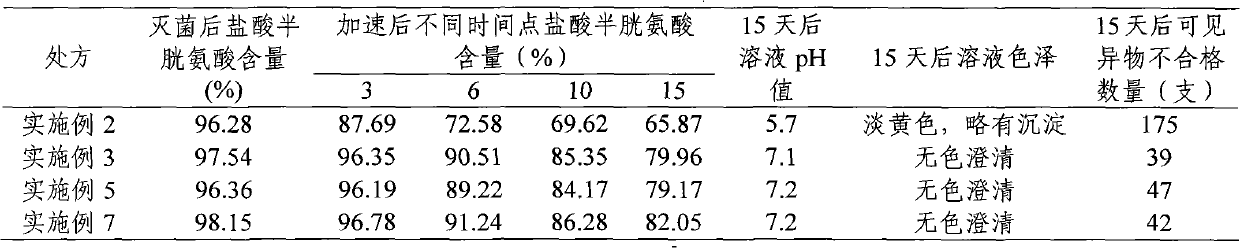
Embodiment 2
[0061] Prescription: Monoammonium Glycyrrhizinate (equivalent to Glycyrrhizin 40g) 53g
[0062] Glycine 400g
[0063] Cysteine Hydrochloride 20g
[0065] Edetate Calcium Sodium 1g
[0066] Appropriate amount of sodium hydroxide
[0067] Add water for injection to 20000ml
[0068]
[0069] Make 1000 pieces, each 20ml
[0070] Preparation process: Weigh the prescribed amount of monoammonium glycyrrhizinate, glycine, and cysteine hydrochloride, put them in a sterilized container, add 2000ml of water for injection, stir to dissolve in a water bath at 37°C, and then add sodium bisulfite 1. Calcium and sodium edetate, stir to dissolve, add water to 2500ml, adjust the pH value to about 7.2 with 1.0mol / L sodium hydroxide solution, add the prescribed amount of activated carbon, keep stirring at 80°C for 40 minutes, and use aseptic pumping Decarburize by filtering with a filter device, add 20,000 ml o...
Embodiment 3
[0072] Prescription: Monoammonium Glycyrrhizinate (equivalent to Glycyrrhizin 40g) 53g
[0073] Glycine 400g
[0074] Cysteine Hydrochloride 20g
[0075] L-Arginine 35g
[0076] Disodium hydrogen phosphate-2H 2 O 712.2g
[0077] Sodium dihydrogen phosphate-2H 2 O 624.2g
[0078] Sodium chloride 170.8
[0079] Add water for injection to 20000ml
[0080]
[0081] Make 1000 pieces, each 20ml
[0082] Preparation process: Weigh monoammonium glycyrrhizinate, glycine, cysteine hydrochloride, L-arginine, sodium dihydrogen phosphate, disodium hydrogen phosphate, and sodium chloride in a sterilized container, add Water for injection 5000ml, in a water bath at 40±5°C, stir to dissolve it, then add activated carbon for injection, keep stirring at 60±1°C for about 30 minutes, filter, filter the filtrate with a 0.22um microporous membrane, and replenish water for injection To the prescribed amount, sub-package, circulation steam steriliza...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com