Water-soluble cationic iridium complex phosphorescence probe and preparation method
A water-soluble cation and iridium complex technology, applied in the field of phosphorescent chemical probes, can solve the problems of cell damage, no hydrophilic group, high rigidity, etc., and achieve the effect of eliminating interference and improving detection sensitivity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
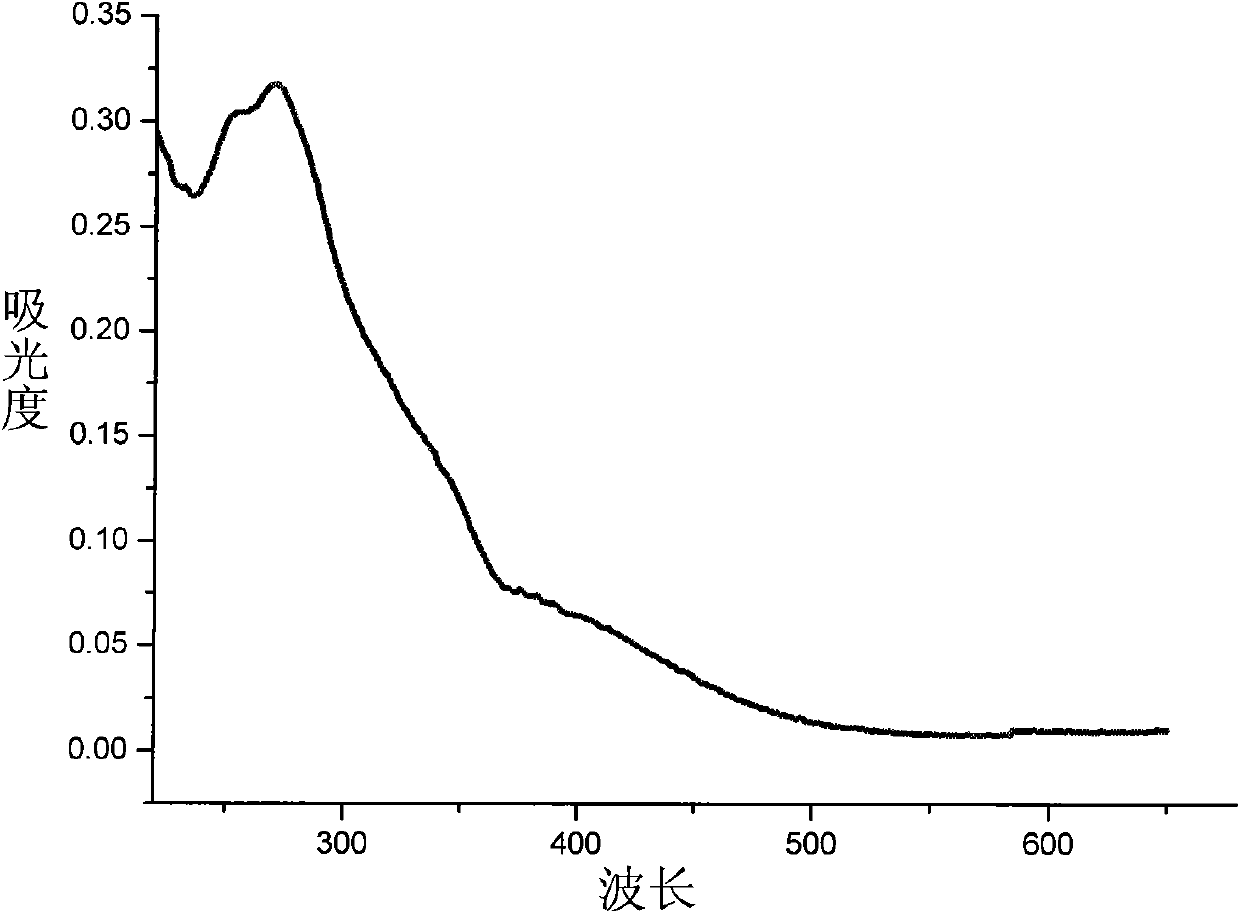
Image
Examples
Embodiment 1
[0052] 2-Phenylpyridine iridium dichloro bridge complex (Ir 2 (ppy) 4 Cl 2 ) preparation: weigh IrCl 3 ·3H 2 O (5.52mmol) and 2,4-difluorophenylpyridine (11.04mmol) were added in the three-necked flask, and then the mixed solution of 2-ethylene glycol ether and water was added, and the volume ratio of the two was 4:1; then The reaction mixture was heated to 110° C. and stirred for 30 hours. After the reaction was completed, it was cooled to room temperature (25° C.), and a yellow precipitate was formed. The resulting precipitate was washed with water and ethanol, respectively, and dried in vacuo to obtain a bright yellow solid 2,4-difluorophenylpyridine iridium dichloro bridge complex, Ir 2 (dfppy) 4 Cl 2 .
Embodiment 2
[0054] The preparation of 3,8-diethynyl o-phenanthroline: under nitrogen protection, 3,8-dibromophenanthroline 10.68mmol, Pd (pph 3 ) 4 0.54mmol, 5mol%, CuI 1.50mmol, 14mol% were mixed in 160mL of degassed tetrahydrofuran and triethylamine degassed (v / v 8:1) mixed solution, and then added 42.72mmol of trimethylsilylacetylene. After heating to reflux for 18 hours, it was cooled, and the solid was removed by filtration, and an appropriate amount of dichloromethane was added to the filtrate. Then it was washed with water three times, dried over anhydrous magnesium sulfate, and the solvent was spun out; the obtained solid was dissolved in 90 mL of methanol, and excess potassium fluoride was added, and stirred at room temperature for 25 hours. After the reaction, it was washed with water, extracted with dichloromethane, dried over anhydrous magnesium sulfate, and purified by spin-drying column chromatography to obtain a white solid product.
[0055] 1 H NMR: (400MHz, CDCl 3 ), ...
Embodiment 3
[0057] Preparation of 3,8-diethynyl-phenanthroline iridium 2-phenylpyridine complex: Accurately weigh 0.25 mmol of 2-phenylpyridine-iridium dichloro bridge complex and 3,8-diethynyl-phenanthroline Add 0.75mmol into a three-neck flask; add 60mL of a mixed solution of dichloromethane and methanol, the volume ratio of which is 5:1; heat to 45°C, and stir for 26 hours. After the reaction, the reaction solution was spin-dried, and the red solid product was obtained by chromatography.
[0058] 1 H NMR: (400MHz, CDCl 3 ), δ(ppm): 8.98(s, 2H), 8.64(s, 2H), 8.19(d, 2H, J=1.6Hz), 7.95(d, 2H, J=8.4Hz), 7.78(t, 2H , J=7.6Hz), 7.73(d, 2H, J=6.8Hz), 7.32(d, 2H, J=5.6Hz), 7.08(t, 2H, J=7.4Hz), 6.97(m, 4H), 6.35 (d, 2H, J = 7.2 Hz), 3.47 (s, 2H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com