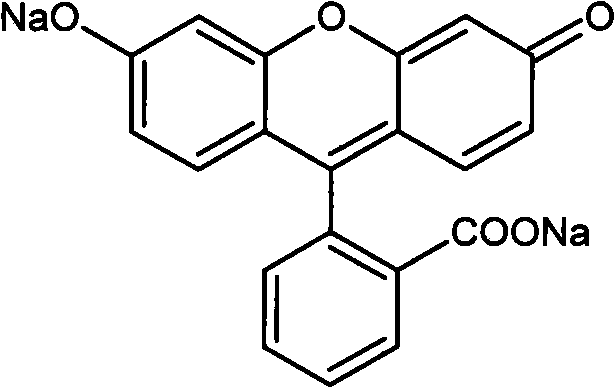
Preparation method of high-purity fluorescein sodium
A technology of sodium fluorescein and fluorescein, applied in the direction of organic chemistry, can solve the problems of increasing production costs, increasing preparation costs, and the quality of pharmaceutical-grade finished products cannot be guaranteed.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
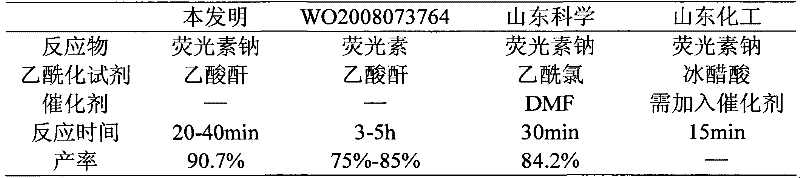
[0025] Take 50.0 g of crude sodium fluorescein and 230 ml of acetic anhydride in a round bottom flask, and heat to reflux for 30 min. TLC inspection showed that sodium fluorescein had completely reacted. Cool down to below 25°C in an ice bath, and keep warm for 20 minutes to fully crystallize. Add 100ml of water, stir, and keep the temperature below 25°C. After dropping, stir for 10 minutes, filter with suction, wash the filter cake with water until the filtrate is colorless, and dry the filter cake to obtain crude diacetylfluorescein in light yellow crystals, yield: 90.7%
[0026] TLC conditions: Chloroform: Methanol: Ammonia = 30:15:1
[0027] Table 1 present invention and prior art acetylation reaction contrast
[0028]
Embodiment 2
[0030] Obtain 45.0g of crude acetyl fluorescein, add 200ml of dichloromethane, 40ml of petroleum ether (60-90), heat to reflux, cool and add 1.0g of activated carbon, reflux for 30 minutes, filter while it is hot, the mother liquor precipitates solid at room temperature, suction filter, and dry. About 40 g of the diacetylated compound in the form of white needle crystals was obtained. Yield: 89%.
Embodiment 3
[0032] Take 42.0g of refined diacetylated fluorescein and 160ml of distilled water in a reaction bottle, add NaOH solution (20.0g of NaOH, 40ml of distilled water), heat, and keep the reaction solution at 80°C for 40 minutes. Add 2.0 g of activated carbon, stir for 20 minutes, cool to room temperature, and filter. The filter cake was washed with 20ml of water and sucked dry. The filtrate was cooled, and dilute hydrochloric acid was slowly added dropwise to adjust the pH to 2.0 to obtain a red precipitate. After filtering, the filter cake was fully washed with distilled water, drained, and the filter cake was vacuum-dried to constant weight to obtain fluorescein. Yield: 97%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com