Method for preparing carbon-coated superparamagnetic ferroferric oxide gel
A technology of ferric oxide and superparamagnetism, which is applied in the direction of iron oxide/iron hydroxide, magnetic properties of inorganic materials, oxides of ferrous iron, etc. It can solve the problems of complicated process, high requirement of reaction conditions, poor stability, etc. Problems, to achieve the effect of simple process, high chemical and colloidal stability, and overcome the high cost of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Embodiment 1. prepares uniform ferric oxide colloidal ball
[0034] Take 0.3g of ferrocene, dissolve it in 30.0mL of acetone, ultrasonically disperse it, then add 1.5ml of 30% hydrogen peroxide dropwise, stir magnetically at 1000r / min for 30 minutes, and then transfer the solution to a volume of 40ml In an autoclave, seal and heat to 240°C, keep it warm for 72 hours, and then cool to room temperature to obtain a black powdery solid; then wash the sample 3 times with acetone and ethanol to remove residual organic matter in the solid powder; then the solid The powder was dried in a vacuum oven at 40°C for 6 hours to obtain the product.
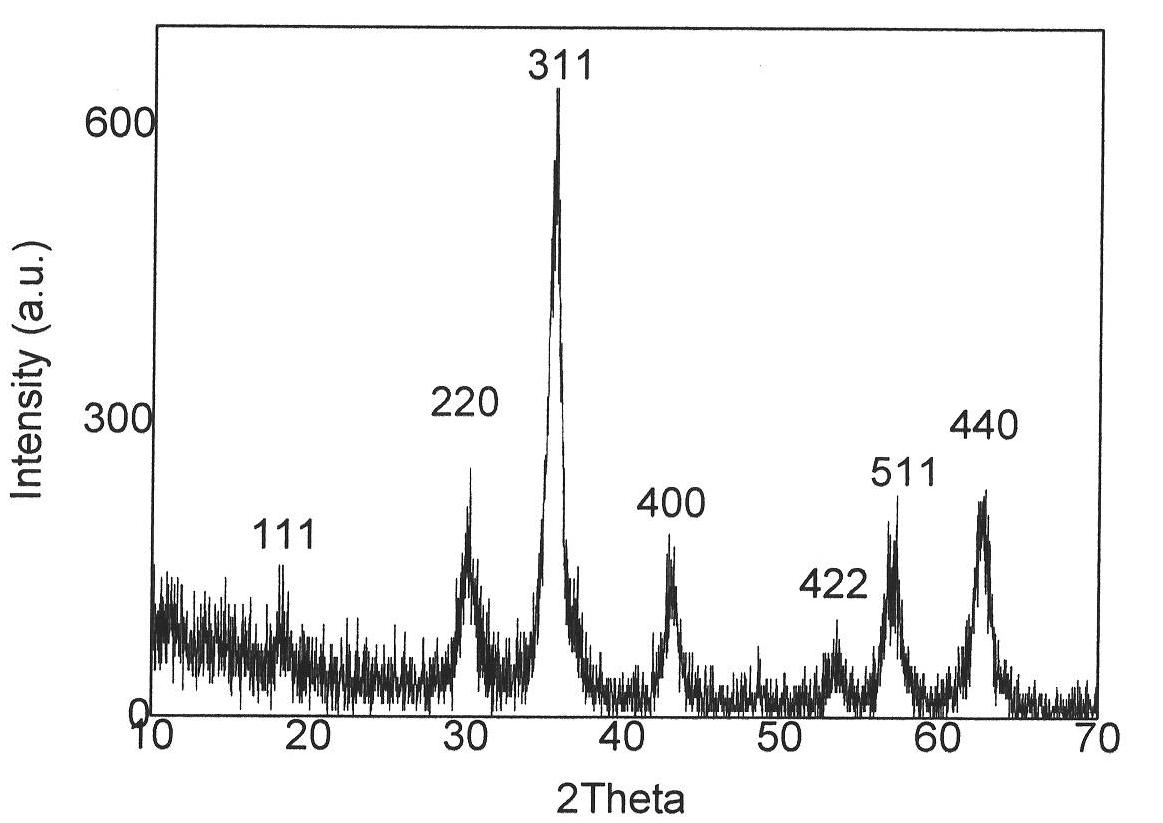
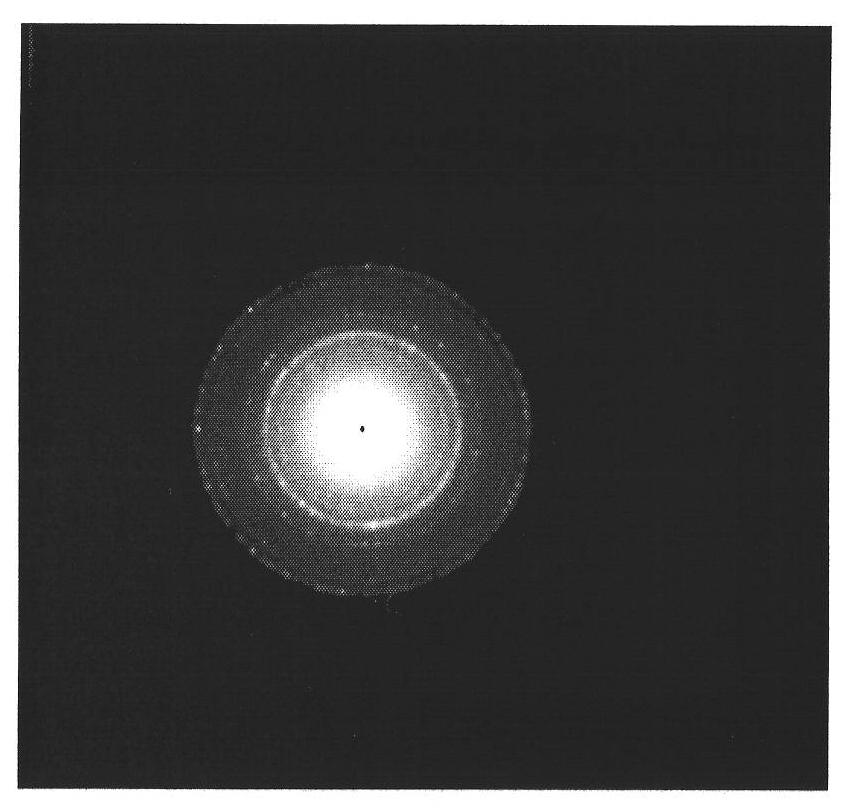
[0035] Such as figure 1 Shown, according to the X-ray diffraction of present embodiment product Figure, all diffraction peak positions correspond to (111), (220), (311), (222), (400), (422), (511), (440) face of ferric oxide respectively, show product as Ferric oxide; By the transmission electron microscope electron diffraction image ...
Embodiment 2
[0036] The influence of the amount of embodiment 2.ferrocene on product
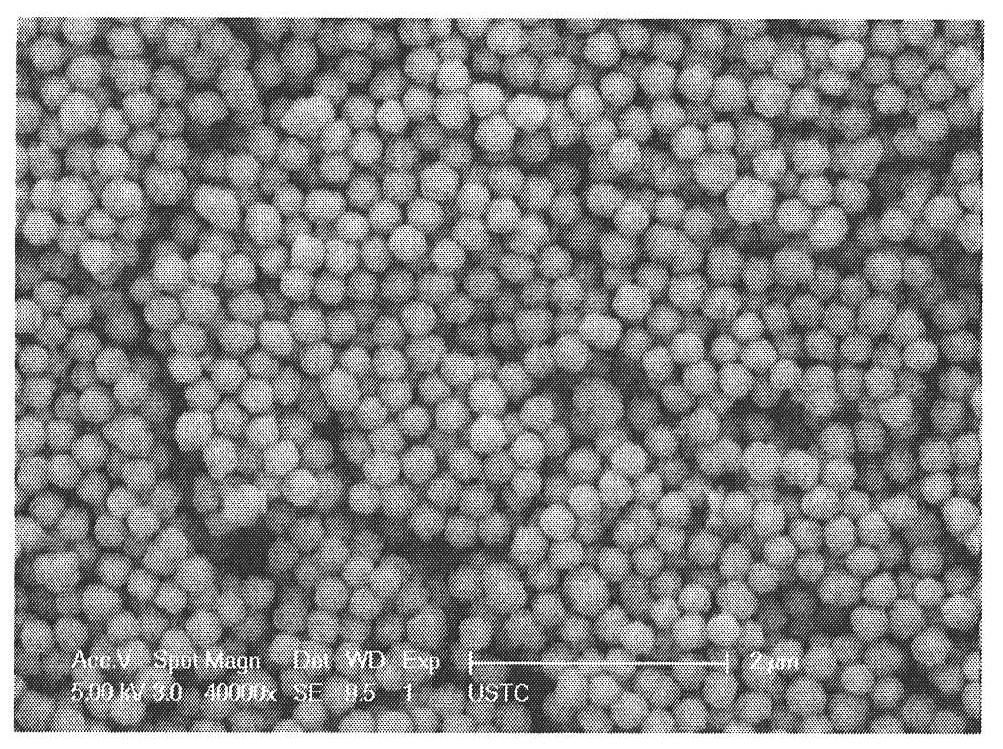
[0037] Take 0.1g and 0.2g of ferrocene, dissolve in 30.0mL of acetone, ultrasonically disperse, then add 1.5ml of 30% hydrogen peroxide dropwise, stir magnetically at 1000r / min for 30 minutes, then transfer the solution to In an autoclave with a capacity of 40ml, seal and heat to 240°C for 72 hours, then cool to room temperature to obtain a black powdery solid; then wash the sample 3 times with acetone and ethanol to remove residual organic matter in the solid powder; then The solid powder was dried in a vacuum oven at 40° C. for 6 hours to obtain the product. The product looks like Figure 8 (0.10g) and Figure 9 (0.20g). Figure 8 with Figure 9 It shows that the smaller the amount of ferrocene, the smaller the thickness of the carbon layer on the surface of ferric oxide nanospheres.
Embodiment 3
[0038] Embodiment 3. The influence of the amount of hydrogen peroxide on product
[0039] Adopt the same method as in Example 1 to configure a portion of the same solution, that is, weigh 0.3g ferrocene, dissolve it in 30.0ml acetone, and only change the amount of hydrogen peroxide added later to 0.5ml, 1.5ml, 3ml , and the other processes are the same. The obtained results were confirmed by X-ray diffraction analysis, all of which were colloidal spheres of ferric oxide. The difference was that colloidal spheres with particle diameters of 90nm, 150nm and 200nm were obtained respectively. A smaller amount of hydrogen peroxide can only obtain small particles with weaker magnetic properties, not ferric oxide, and too much hydrogen peroxide has little effect on the change of particle size, so the amount of hydrogen peroxide is usually between 0.5ml- between 3ml. The resulting product looks like Figure 10 , Figure 11 with Figure 12 shown. Figure 10 , Figure 11 with Fi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com