Preparation method of 2,3,6,7,10,11-substituted benzophenanthrene
A technology of triphenylene and o-dialkoxybenzene, which is applied in the field of liquid crystal materials and organic photoelectric functional materials, can solve the problems of high price and unsuitability for mass production, and achieve easy mass production, shortened reaction time, and reduced costs Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
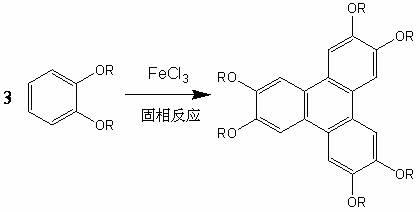
[0030] Put 2mmol0.44g of o-dibutoxybenzene and 8mmol1.30g powder anhydrous ferric chloride into a glass mortar, grind it with a pestle for about 0.5 hours at room temperature, no obvious irritating gas is released in the mortar, The mixture was poured into ethanol containing 5% hydrochloric acid, stirred for 5 minutes, and the filtered precipitate was left to dry to obtain the primary product of 2,3,6,7,10,11-hexa-substituted triphenylene.
[0031] First add 2 mL of dichloromethane to the crude product to dissolve it, then add 10 mL of ethanol for precipitation, collect the precipitate and recrystallize with ethanol to finally obtain 0.41 g of a purple flaky solid with a yield of 93%.
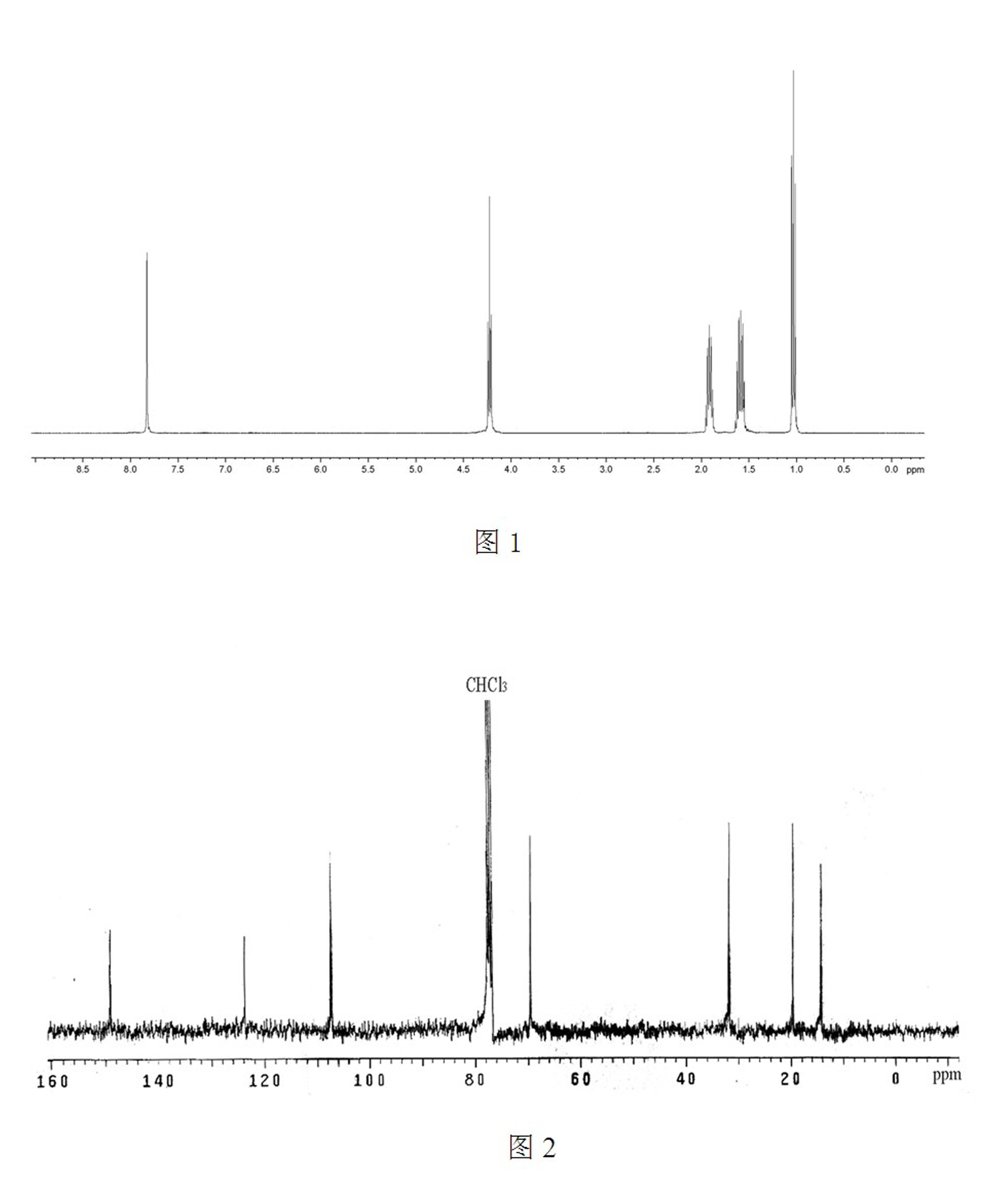
[0032] Such as figure 1 , the chemical structure of the product was confirmed by H NMR and C NMR spectra.
Embodiment 2
[0034] Put 2mmol0.44g of o-dibutoxybenzene and 4mmol0.65g of powdered anhydrous ferric chloride into a glass mortar, grind with a hammer at room temperature for 0.5 hours, no obvious irritating gas is released in the mortar, The mixture was poured into ethanol containing 2% hydrochloric acid, stirred for 5 minutes, and the filtered precipitate was left to dry to obtain the primary product of 2,3,6,7,10,11-hexa-substituted triphenylene.
[0035] First add 2 mL of chloroform to the crude product to dissolve it, then add 10 mL of ethanol for precipitation, collect the precipitate and recrystallize with ethanol to finally obtain 0.25 g of a purple flaky solid with a yield of 56%.
[0036] The chemical structure of the product was confirmed by NMR spectrum and Fourier transform infrared spectrum.
Embodiment 3
[0038] Put 2mmol0.44g of o-dibutoxybenzene and 6mmol0.98g of powdered anhydrous ferric chloride into a glass mortar, grind with a mortar for 40min at room temperature, no obvious irritating gas is released in the mortar, and put The mixture was poured into ethanol containing 3% hydrochloric acid, stirred for 5 minutes, and the filtered precipitate was left to dry to obtain the primary product of 2,3,6,7,10,11-hexa-substituted triphenylene.
[0039] Add 2 mL of chloroform to the crude product to dissolve it, and then add 10 mL of ethanol for precipitation. After the precipitate was collected, it was recrystallized with ethanol to finally obtain 0.36 g of a purple flaky solid with a yield of 82%.
[0040] The chemical structure of the product was confirmed by NMR spectrum and Fourier transform infrared spectrum.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com