New process for preparing epoxide intermediate of Bicalutamide by utilizing air oxidation
A technology of bicalutamide and epoxides, which is applied in the new technology field of epoxide intermediates for the preparation of bicalutamide by air oxidation, which can solve the high requirements for production equipment and operator protection, and the high cost of m-CPBA , peroxide explosion hazard and other issues, to achieve the effect of improved atom economy, no explosion hazard, and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
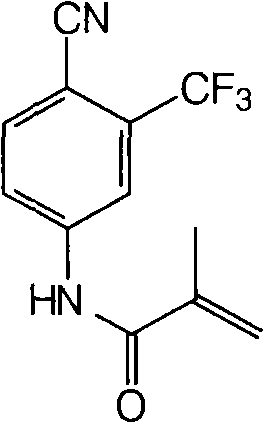
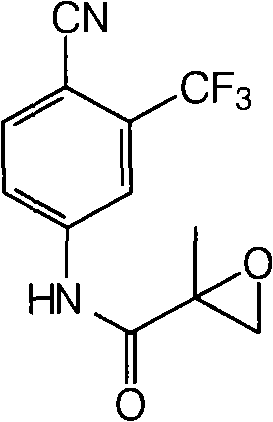
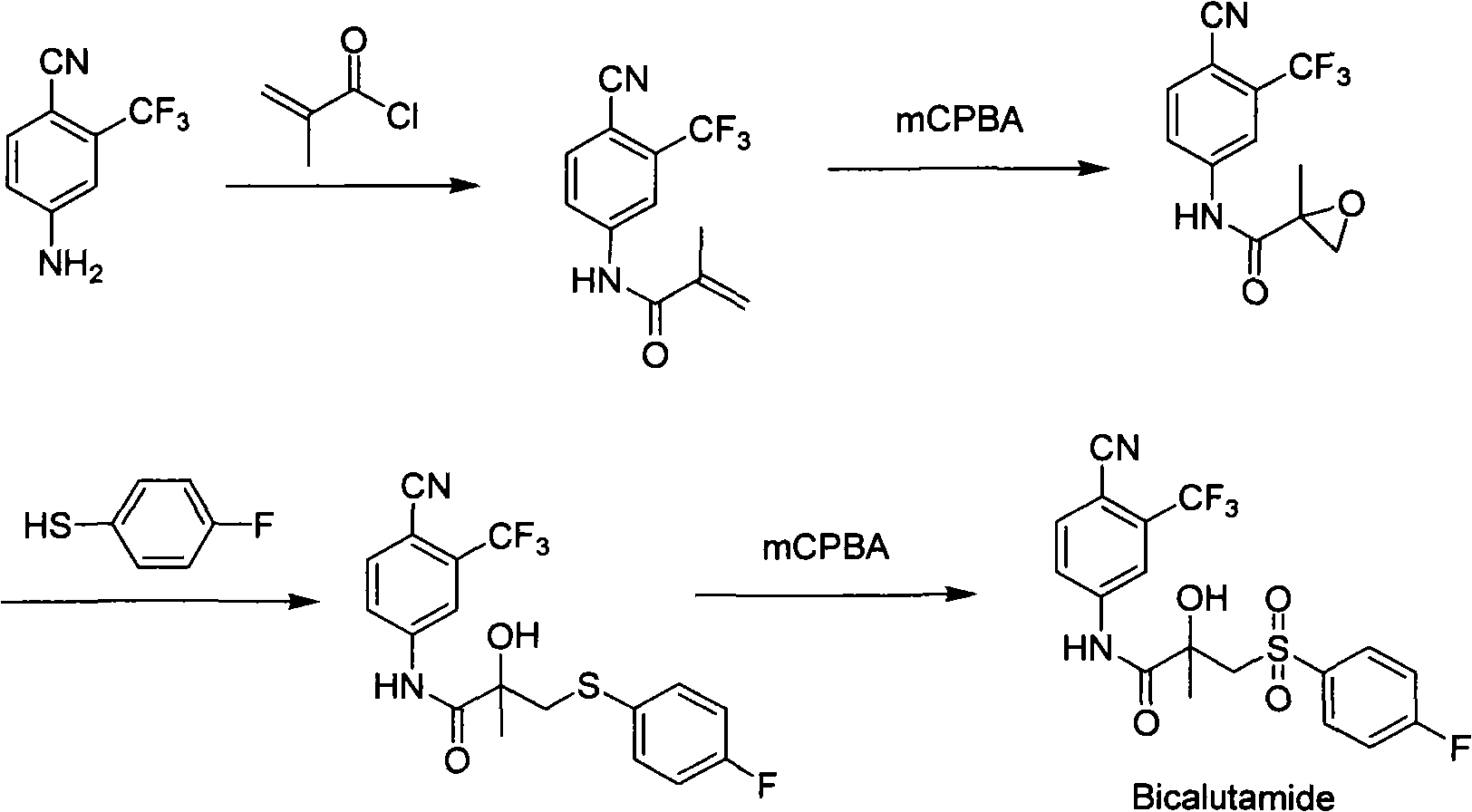
[0031] Dissolve N-(4-cyano-3-trifluoromethylphenyl)-2-methacrylamide (6.35g, 0.025mol) in the solvent ethyl acetate (80ml), add isobutyraldehyde with stirring at room temperature (5.4g, 6.8ml, 0.075mol), catalyst Fe(acac) 3 (0.05g), free radical initiator 20% peroxyacetic acid aqueous solution (0.1ml). Keep the temperature at 25-30°C and react overnight. TLC detects the disappearance of the raw materials. In order to separate the obtained product from the reaction system, water (80ml) was added, the saturated sodium bicarbonate solution was adjusted to pH=7-8, shaken, stand still for separation, and the aqueous layer was extracted with ethyl acetate (2×50ml). The organic layers were combined, dried over anhydrous sodium sulfate, and evaporated to dryness under reduced pressure to obtain an oily substance. It was recrystallized from isopropyl ether to obtain 5.63 g of white bicalutamide epoxy compound crystals. The yield was 83.4%, and the HPLC content was 98.8%.
Embodiment 2
[0033] Dissolve N-(4-cyano-3-trifluoromethylphenyl)-2-methacrylamide (6.35g, 0.025mol) in the solvent dichloromethane (80ml), add benzaldehyde ( 6.6g, 0.075mol), catalyst Co(acac) 3 (0.05g), free radical initiator 30% aqueous hydrogen peroxide solution (0.1ml). Keep the temperature at 25-30°C and react for 20h. TLC detects the disappearance of the raw materials. In order to separate the obtained product from the reaction system, water (80ml) was added, saturated sodium bicarbonate solution was adjusted to pH=7-8, shaken, stood still to separate the layers, and the aqueous layer was extracted with dichloromethane (2×50ml). The organic layers were combined, dried over anhydrous sodium sulfate, and evaporated to dryness under reduced pressure to obtain an oily substance. It was recrystallized from isopropyl ether to obtain 5.91 g of white bicalutamide epoxy compound crystals. The yield was 87.6%, and the HPLC content was 99.3%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com