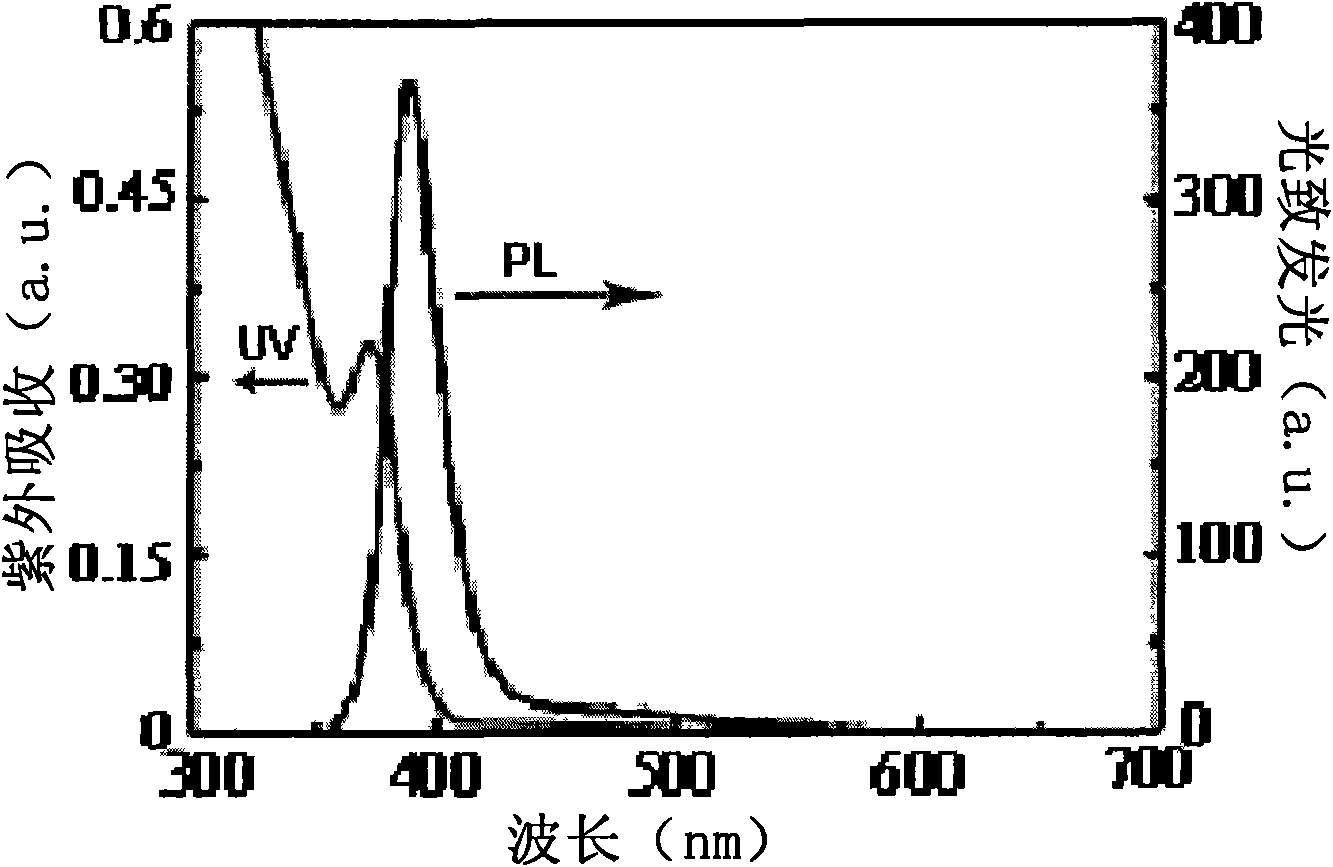
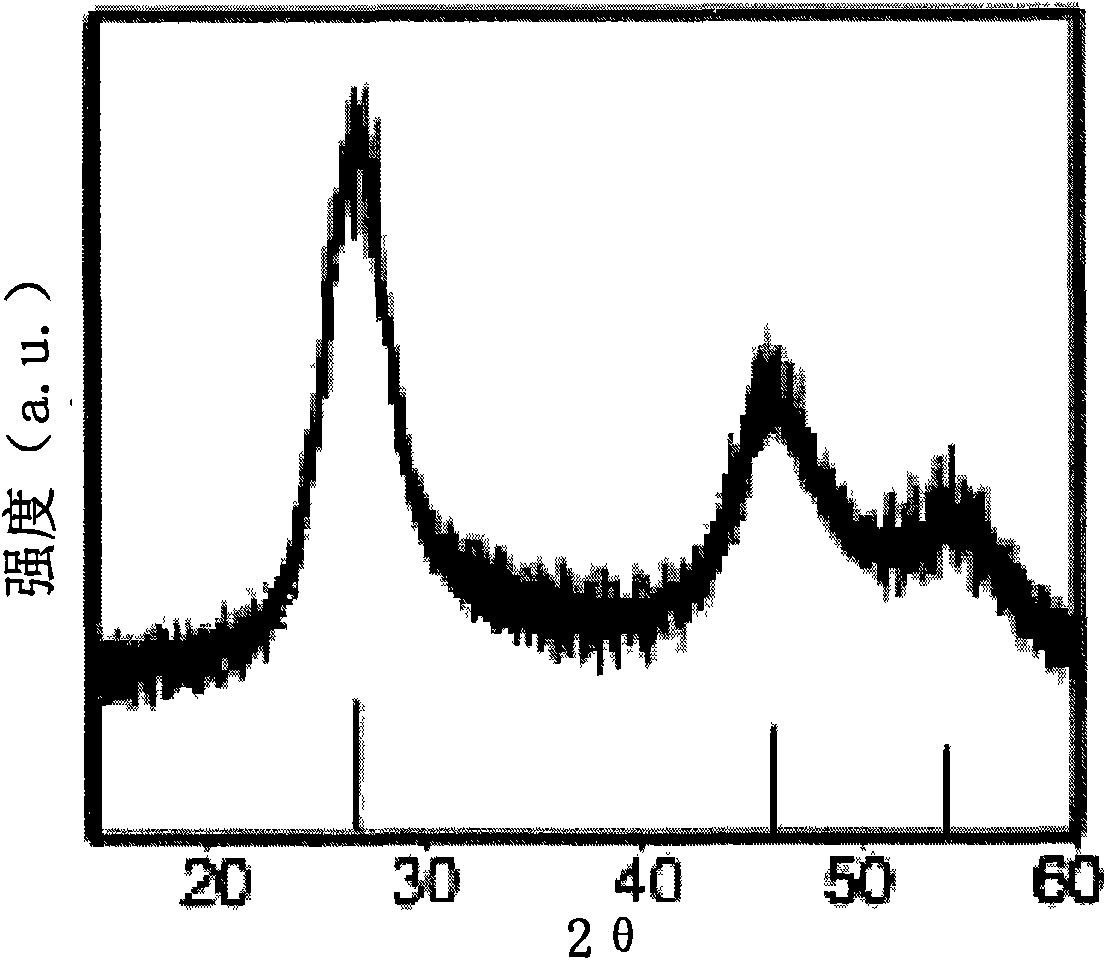
Microwave preparation method of water soluble ZnSe quantum dots
A quantum dot, water-soluble technology, applied in chemical instruments and methods, solution from liquid solvent at room temperature, single crystal growth, etc., can solve the problems of low fluorescence quantum efficiency and difficult preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0030] The microwave preparation method of water-soluble ZnSe quantum dots provided by the invention, the specific steps are as follows:
[0031] 1. Preparation of sodium selenium hydride NaHSe solution as selenium source: sodium borohydride NaBH with a molar ratio of 15:1 to 20:1 4 and selenium powder Se are placed in water, and left to react at room temperature for 24-60 hours to obtain NaHSe solution; Glutathione GSH solution, adjust the pH value of the solution to 8-12, inject NaHSe solution to obtain the ZnSe precursor solution, and store it under nitrogen protection; 3. Microwave the ZnSe precursor solution to obtain water-soluble ZnSe quantum dots, wherein the microwave heating conditions are: microwave power 50W-300W, heating time 1 minute-30 minutes, heating temperature 70-120 degrees Celsius, to obtain water-soluble ZnSe quantum dots with high fluorescence quantum efficiency.
[0032] Zinc salt or zinc oxide and hydroxide described in the present invention include: ...
Embodiment 1
[0049] Preparation of water-soluble ZnSe quantum dots I
[0050] (1) Preparation of NaHSe
[0051] 23.9 mg NaBH 4 Mix with 23.6 mg of Se powder, dissolve in 3 ml of ultrapure water, then keep the solution away from light, and store it at room temperature. 2 . After about 30 hours, the black Se powder disappeared and white Na appeared at the bottom of the bottle. 2 B 4 o 7 Precipitation, the supernatant is NaHSe solution, and the clear solution is separated for the preparation of ZnSe nanocrystals.
[0052] (2) Preparation of ZnSe precursor solution
[0053] 17.2 mg ZnCl 2 Dissolve in 100 ml of water, add 8.6 microliters of glutathione, adjust pH=11 with 0.5 mol / L NaOH solution, ZnCl 2 Mix the solution with GSH under vigorous stirring with N 2 The gas was deoxygenated for 30 minutes, and then 2 ml of NaHSe solution was injected as the ZnSe precursor solution.
[0054] (3) Preparation of ZnSe quantum dots by microwave irradiation
[0055] Take 20 ml of the ZnSe precur...
Embodiment 2
[0059] Preparation of water-soluble ZnSe quantum dots II
[0060] (1) Preparation of NaHSe
[0061] 17.5 mg NaBH 4 Mix with 20.1 mg of Se powder, dissolve in 3 ml of ultrapure water, then keep the solution away from light, and store it at room temperature. 2 . After about 48 hours, the black Se powder disappeared and white Na appeared at the bottom of the bottle 2 B 4 o 7 Precipitation, the supernatant is NaHSe solution, and the clear solution is separated for the preparation of ZnSe nanocrystals.
[0062] (2) Preparation of ZnSe precursor solution
[0063] 35.5 mg ZnCl 2 Dissolve in 100 ml of water, add 18.7 microliters of glutathione, adjust pH=8 with 0.5 mol / L NaOH solution, ZnCl 2 Mix the solution with GSH under vigorous stirring with N 2 The gas was deoxygenated for 30 minutes, and then 3 ml of NaHSe solution was injected as the ZnSe precursor solution.
[0064] (3) Preparation of ZnSe quantum dots by microwave irradiation
[0065] Take 20 ml of the ZnSe precur...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com