Medical use of inhibitors of glutaminyl and glutamate cyclases
A technique for inhibitors of glutaminyl cyclase and aminopeptidase, applied in the field of effector screening
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0279] Embodiment 1: the preparation of people and papaya QC
[0280] Host Strains and Media
[0281] Pichia pastoris strain X33 (AOX1, AOX2) for human QC expression was grown, transformed and analyzed according to the manufacturer's instructions (Invitrogen). The media required for P. pastoris, buffered glycerol (BMGY) complex or methanol (BMMY) complex medium and fermentation basal salt medium, were prepared according to the manufacturer's recommendations.
[0282] Molecular cloning of a plasmid vector encoding human QC
[0283] All cloning steps are performed using standard molecular biology techniques. The vector pPICZαB (Invitrogen) was used for expression in yeast. The pQE-31 vector (Qiagen) was used to express human QC in E. coli. The cDNA for the mature QC starting at codon 38 was fused in-frame to a plasmid-encoded 6×histidine tag. After amplification and subcloning using primers pQCyc-1 and pQCyc-2 (Table 1), the fragment was inserted into the expression vec...
Embodiment 2
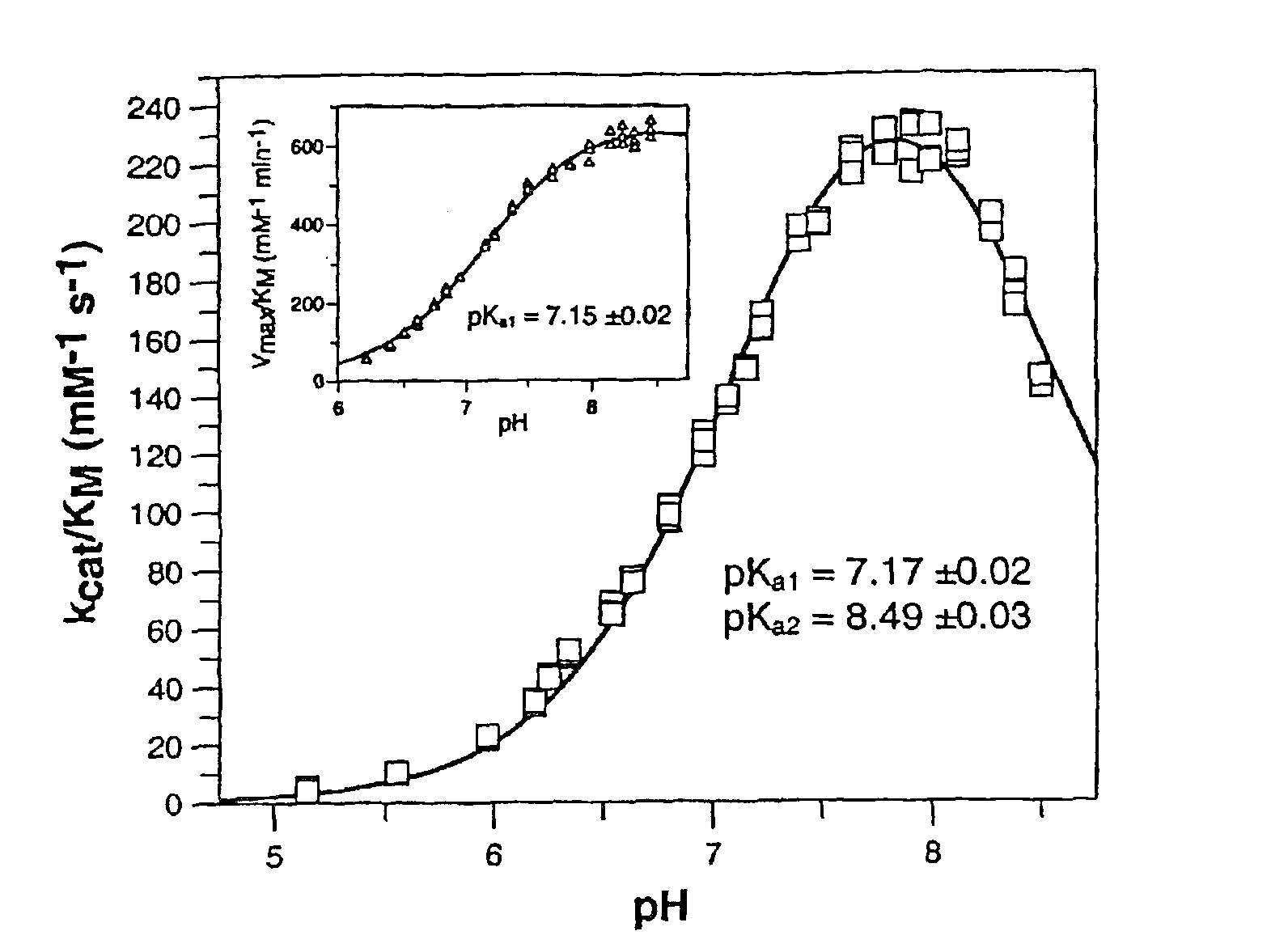
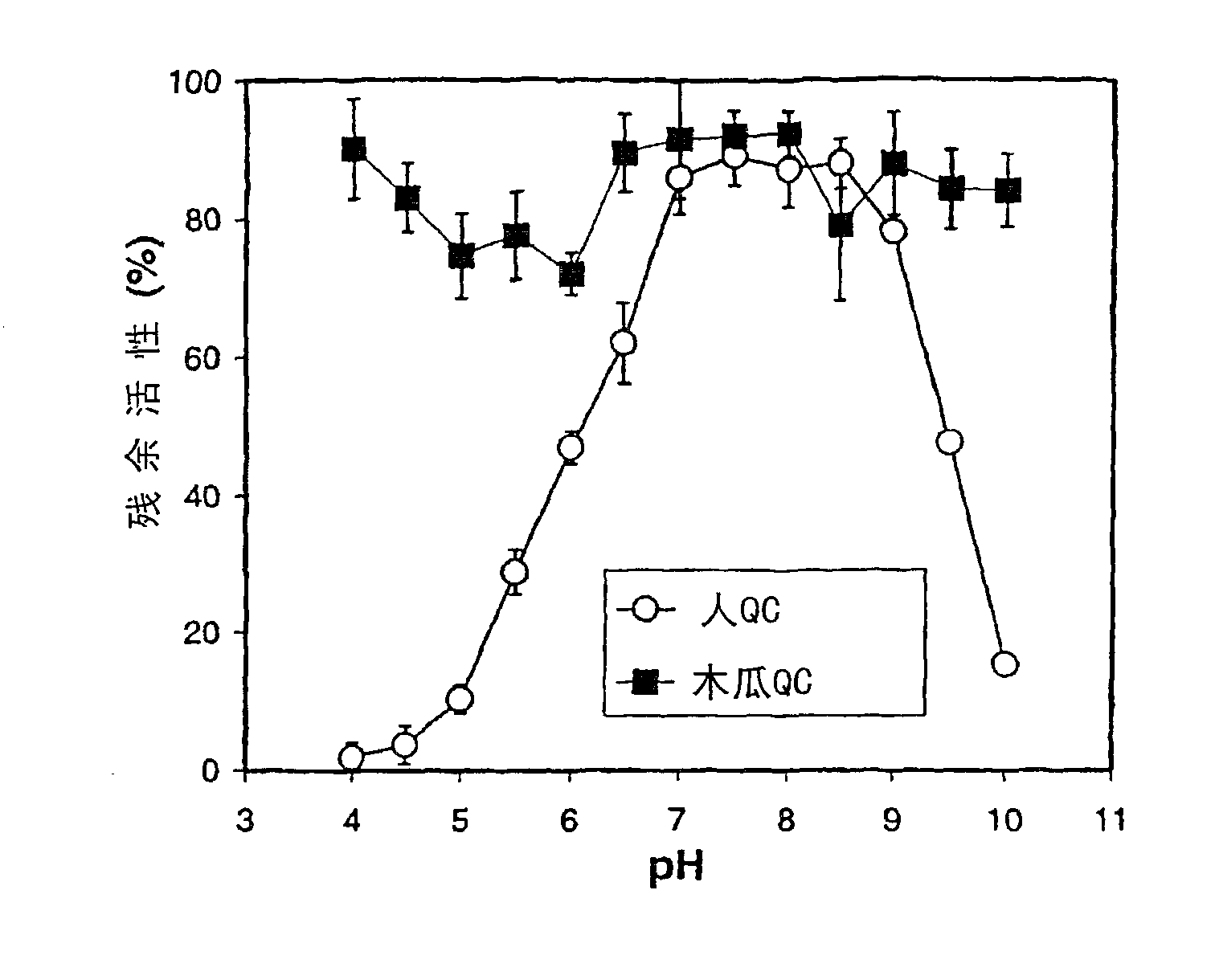
[0296] Example 2: Determination of glutaminyl cyclase activity
[0297] Fluorescence analysis
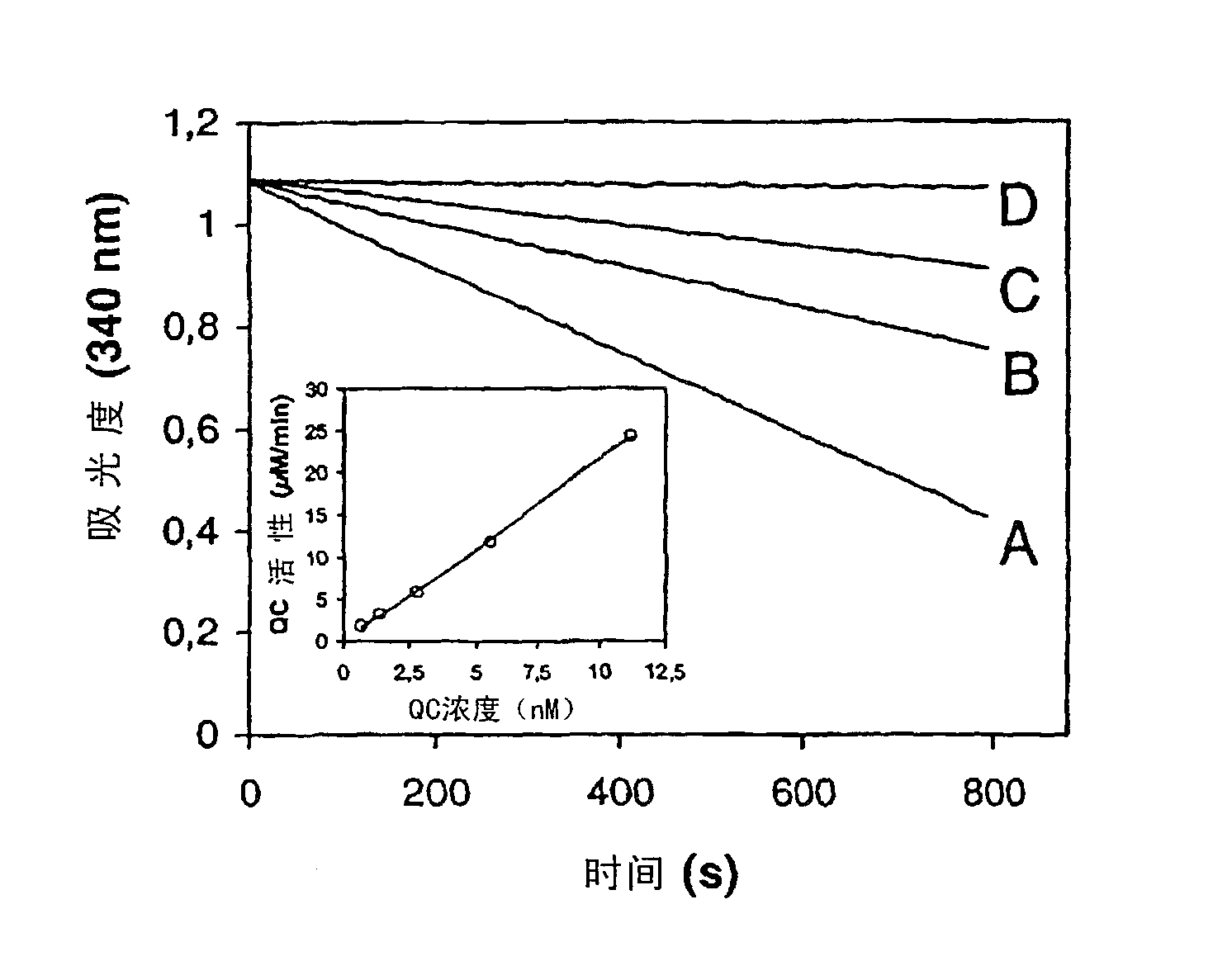
[0298] All assays were performed at 30°C using a BioAssay Reader HTS-7000Plus microplate reader (Perkin Elmer). QC activity was assessed fluorimetrically using H-Gln-βNA. The sample was prepared with 0.25 U pyroglutamyl aminopeptidase (Unizyme, Denmark) and appropriately diluted QC aliquots in a final volume of 250 μl. The excitation / emission wavelengths are 320 / 410nm. The assay reaction was initiated by the addition of glutaminyl cyclase. QC activity was determined from a β-naphthylamine standard curve under analytical conditions. One unit is defined as the amount of QC that catalyzes the generation of 1 μmol pGlu-βNA from H-Gln-βNA per minute under the stated conditions.
[0299] In the second fluorescence assay, the activity of QC was determined using H-Gln-AMC as substrate. Reactions were performed at 30°C using a NOVOStar microplate reader (BMG labtechnologies). The ...
Embodiment 3
[0305] Embodiment 3: MALDI-TOF mass spectrometry analysis
[0306] Matrix-assisted laser desorption / ionization mass spectrometry was performed using a Hewlett-Packard G2025LD-TOF System with a linear time-of-flight analyzer. The device is equipped with a 337nm nitrogen laser, a voltage acceleration source (5kV) and a 1.0m flight tube. The detector was in positive ion mode, and a LeCroy 9350M digital storage oscilloscope connected to a personal computer was used to record and filter the signal. Samples (5 μl) were mixed with an equal volume of matrix solution. For the matrix solution, we used an aqueous solution (1 / 1, v / DHAP / DAHC prepared in v). A small volume (=1 μl) of matrix-analyte-mixture was transferred onto the probe tip and evaporated immediately in a vacuum chamber (Hewlett-Packard G2024A sample preparation accessory) to ensure fast and uniform sample crystallization.
[0307] For long-term determination of Glu 1 For cyclization, Aβ-derived peptides were incubate...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com