Clean synthesis method for di-tert-butyl disulfide
A technology of di-tert-butyl disulfide and synthesis method, which is applied in the preparation of hydrogenated polysulfide/polysulfide, organic chemistry, etc., can solve the problems of high cost, difficult recovery of catalyst, high toxicity of solvent, etc., and achieve the reaction time Short, recycled reusable, inexpensive effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
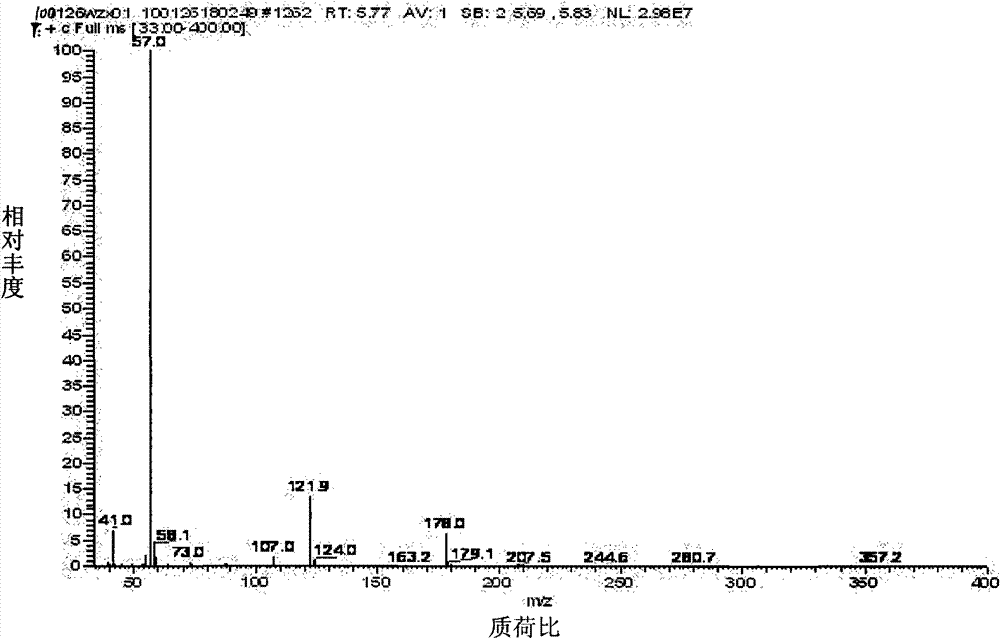
[0018] Add 2.18mmol of catalyst CuCl, 100mL of acetone, 867mmol of tert-butyl mercaptan (t-Bu-SH) into the reaction vessel, and add 54g of 30% (mass fraction of hydrogen peroxide in hydrogen peroxide, the same below) hydrogen peroxide under stirring condition (476.5mmol) and 150mL acetone mixture, [n(CuCl):n(t-Bu-SH):n(H2 o 2 )=2.5: 1000: 550 (representing the molar ratio of each substance, the same below), the volume ratio of the total volume added by the solvent acetone to tert-butyl mercaptan is 2.5: 1], and the control reaction temperature is between 40 ± 5 ° C , react until the detected mercaptan conversion rate reaches 99.8%, stop the reaction. The catalyst was removed by filtration, and then the acetone was removed. After standing for stratification, the oil layer was obtained as di-tert-butyl disulfide, with a selectivity of 94.5%. Mass Spectrum See figure 1 , molecular ion peak 178, base peak 57.
Embodiment 2
[0020] In reaction vessel, add catalyst CuCl 0.0434mmol, acetone 20mL, add tert-butyl mercaptan 86.7mmol, add the mixed solution of 50% hydrogen peroxide 3.5g (51.5mmol) and 10mL acetone under stirring condition, [n(CuCl):n (t-Bu-SH):n(H 2 o 2 )=0.50: 1000: 594, the volume ratio of the total volume added by the solvent acetone to tert-butyl mercaptan is 3.0: 1], the reaction temperature is controlled at 35 ± 5°C, and the reaction until the detected mercaptan conversion rate reaches 99.6%, Stop responding. The catalyst was removed by filtration, and then the acetone was removed. After standing for stratification, the oil layer was obtained as di-tert-butyl disulfide, with a selectivity of 93.8%. Mass Spectrum See figure 1 .
Embodiment 3
[0022] In the reaction vessel, add catalyst CuCl 0.0606mmol, acetone 20mL, add tert-butyl mercaptan 86.7mmol, add the mixed solution of 75% hydrogen peroxide 2.2g (48.5mmol) and 10mL acetone under stirring condition, [n(CuCl):n (t-Bu-SH):n(H 2 o 2 ) = 0.699: 1000: 560, the volume ratio of the total volume added by the solvent acetone to tert-butyl mercaptan is 3.0: 1], the reaction temperature is controlled at 40 ± 3°C, and the reaction until the detected mercaptan conversion rate reaches 98.4%, That is, the reaction stops. The catalyst was removed by filtration, and then the acetone was removed. After standing for stratification, the oil layer was obtained as di-tert-butyl disulfide, with a selectivity of 93.7%. Mass Spectrum See figure 1 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com