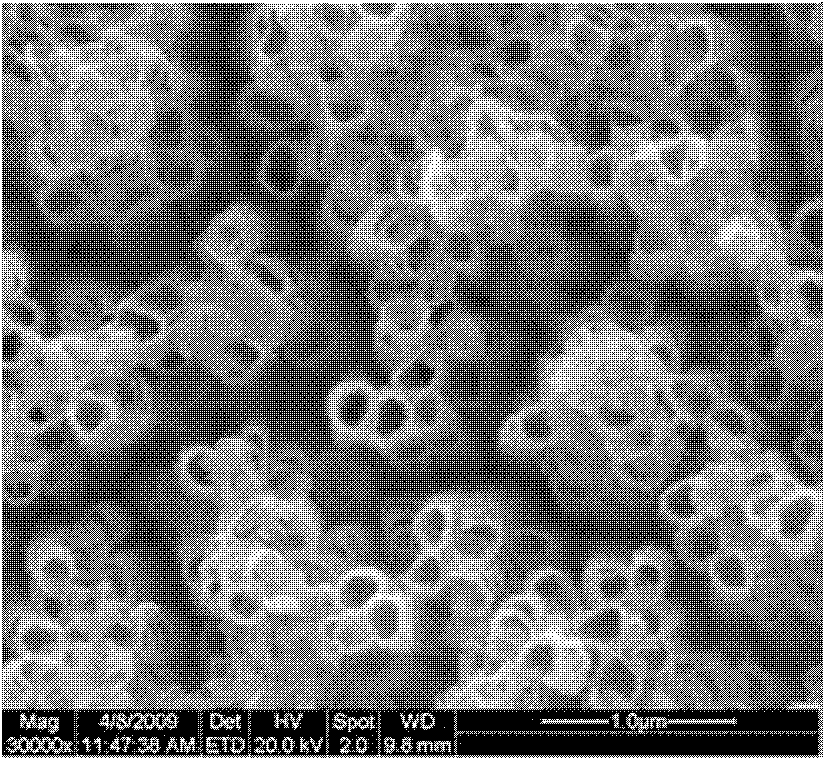
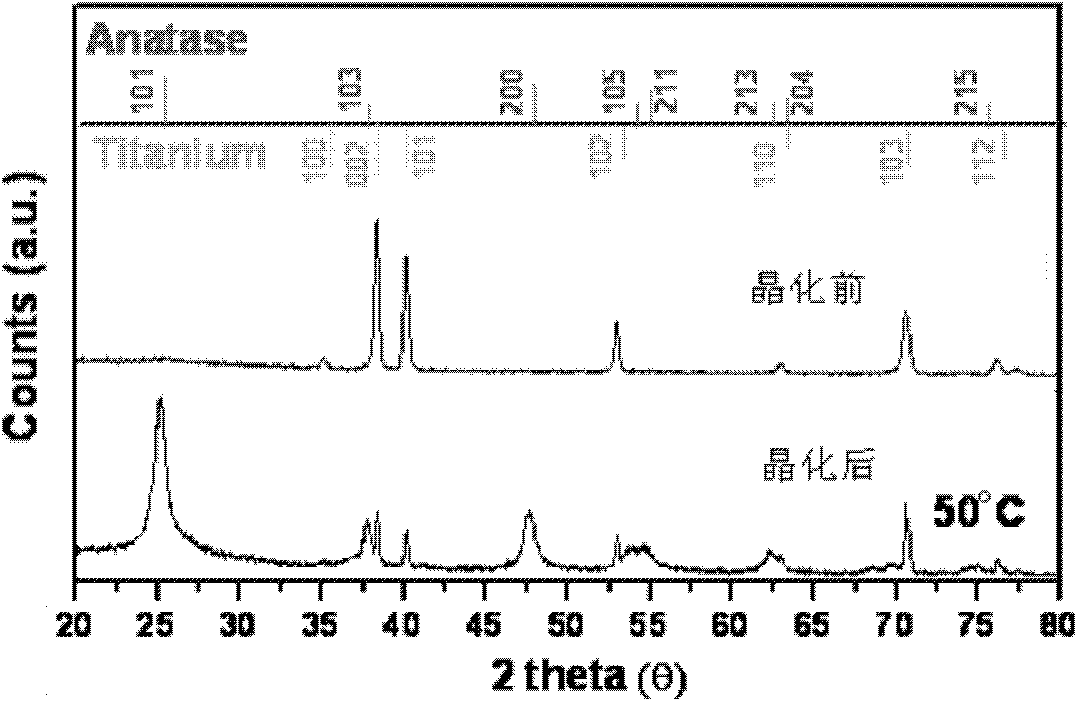
Preparation method of crystallized TiO2 nanotube array
A nanotube array, crystallization technology, applied in the direction of titanium dioxide, titanium oxide/hydroxide, etc., to achieve the effect of short time, low energy consumption and high crystallization success rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] A specific embodiment of the present invention is a low temperature crystallized TiO 2 The method for the nanotube array, its specific practice is:
[0020] a. TiO 2 Fabrication of nanotube arrays
[0021] Put the titanium sheet in acetone for 30 minutes of ultrasonic cleaning. After air drying, put the titanium sheet into an aqueous solution containing 25ml / L of concentrated sulfuric acid, 15ml / L of hydrofluoric acid, and 60ml / L of glacial acetic acid; Electrochemical polishing was carried out by direct current at room temperature for 8 minutes; then the polished titanium sheet was put into an ethylene glycol solution of ammonium fluoride with a weight volume concentration of 0.05% for anodic oxidation, and the cathode used for anodic oxidation was platinum Chip, voltage 60v, reaction time 12 hours;
[0022] b. TiO 2 Crystallization of nanotube arrays
[0023] The resulting TiO 2 The nanotube array is placed in water, and kept in a hydrothermal reaction kettle at...
Embodiment 2
[0028] This example is basically the same as Example 1, the difference is:
[0029] a. TiO 2 Fabrication of nanotube arrays
[0030] Put the titanium sheet in acetone for 35 minutes of ultrasonic cleaning. After air drying, put the titanium sheet into an aqueous solution containing 26ml / L of concentrated sulfuric acid, 14ml / L of hydrofluoric acid, and 50ml / L of glacial acetic acid; Electrochemical polishing was carried out by direct current at room temperature for 15 minutes; then the polished titanium sheet was put into an ethylene glycol solution of ammonium fluoride with a weight volume concentration of 0.07% for anodic oxidation, and the cathode used for anodic oxidation was platinum Chip, voltage 60v, reaction time 10 hours;
[0031] b. TiO 2 Crystallization of nanotube arrays
[0032] The resulting TiO 2 The nanotube array is placed in water, and kept in a hydrothermal reaction kettle at 50° C. for 12 hours to obtain it.
Embodiment 3
[0034] This example is basically the same as Example 1, the difference is:
[0035] a. TiO 2 Fabrication of nanotube arrays
[0036] Put the titanium sheet in acetone for 40 minutes of ultrasonic cleaning. After air drying, put the titanium sheet into an aqueous solution containing 28ml / L of concentrated sulfuric acid, 12ml / L of hydrofluoric acid, and 65ml / L of glacial acetic acid; Direct current electrochemical polishing at room temperature, polishing for 20 minutes; then put the polished titanium sheet into an ethylene glycol solution of ammonium fluoride with a weight volume concentration of 1% for anodic oxidation, and the cathode used for anodic oxidation is platinum Chip, voltage 60v, reaction time 8 hours;
[0037] b. TiO 2 Crystallization of nanotube arrays
[0038] The resulting TiO 2 The nanotube array is placed in water, and kept in a hydrothermal reaction kettle at 50° C. for 14 hours to obtain it.
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com