Beta-alkyl sulfide substituted vinyl propionate sulfur compound and preparation method thereof
A technology of vinyl acrylate and vinyl propionate, applied in the chemical field, can solve problems such as residual side reactions and harmful effects, and achieve the effects of wide application range, good yield, and practical industrial application value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
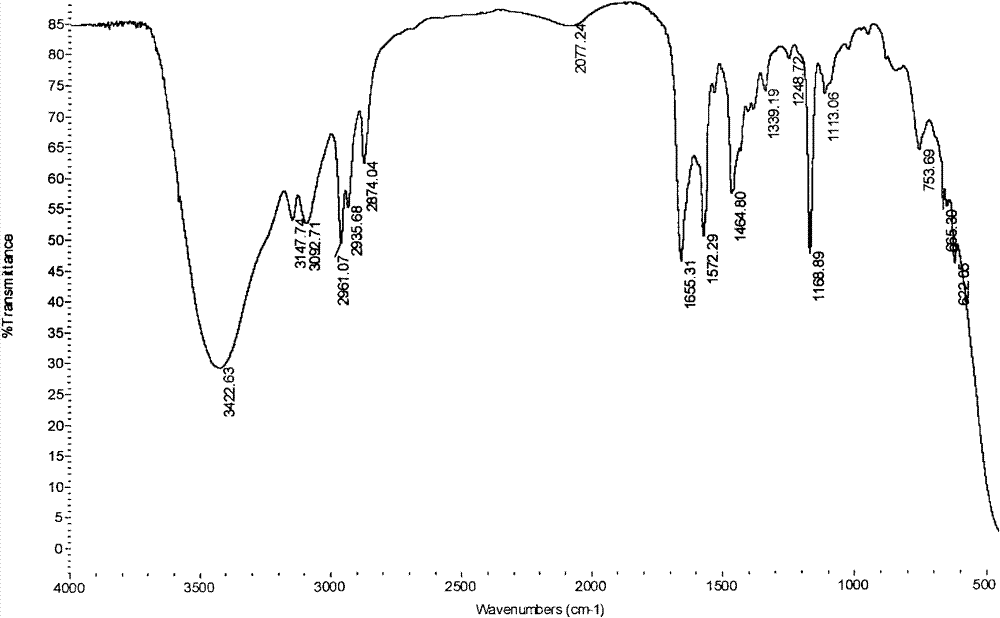
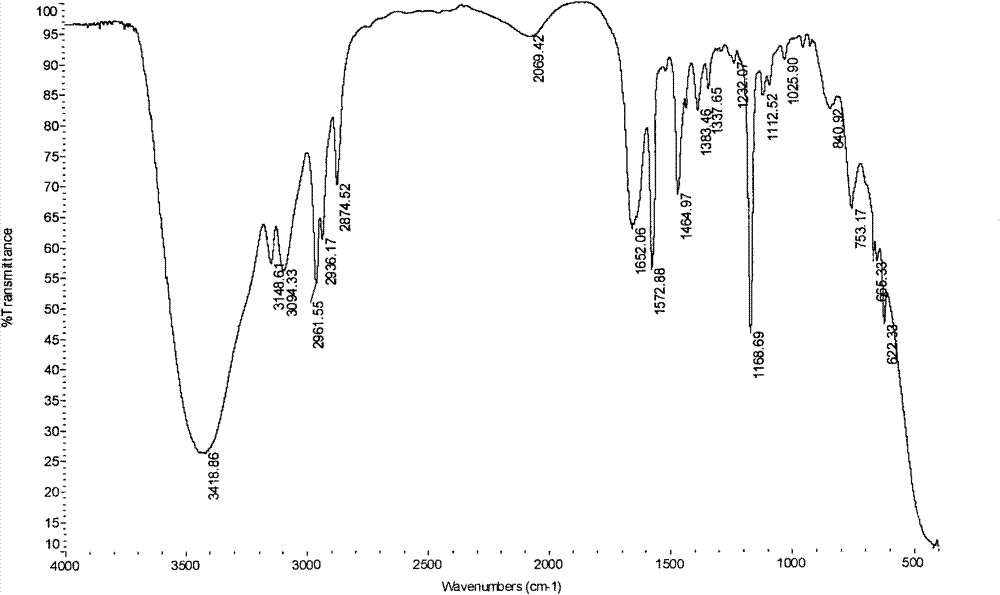
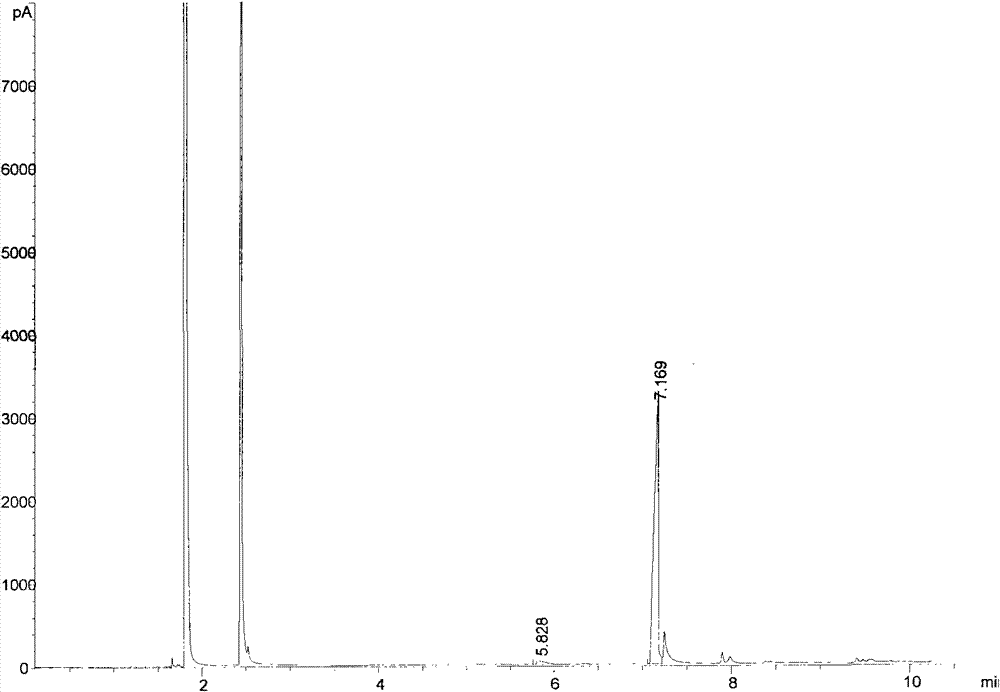
[0057] In a 200mL conical flask with ground mouth, first add 50mL of ionic liquid hydroxide [1-butyl-3-methylimidazole], then add 0.1mol thiophenol to the conical flask, mix well and then add 0.12mol formazan vinyl acrylate at 1kg / cm 2 Pressure, 20°C constant temperature shaking incubator, shake at a speed of 200 rev. / min; TLC monitors the reaction process, and it shows that the reaction is complete after 10 minutes, and the components of the reaction solution are detected by GC chromatography, such as image 3 ; image 3 middle t R =5.828 is the chromatographic peak of thiophenol, t R =7.169 is the chromatographic peak of the product; Add 60mL of ethyl acetate to the reaction solution to extract 5 times after the end, after the extracts are combined, the water pump decompression distillation reclaims ethyl acetate, the resulting product yield is 93%, and the infrared spectrum is characterized by Figure 4 , characterized by H NMR spectroscopy as Figure 5 , characterized ...
Embodiment 2
[0064] In a 200ml ground-mouth conical flask, first add 50ml of ionic liquid hydroxide [1-butyl-3-methylimidazole], then add 0.1mol thiophenol to the conical flask, mix well and add 0.12mol croton Acetate vinyl ester, at 1kg / cm 2 Pressure, 20°C constant temperature shaking incubator, shake at a speed of 200 rev. / min; TLC monitors the reaction process, and it shows that the reaction is complete after 10 minutes. After the end, add 60ml of ethyl acetate to the reaction solution for extraction 5 times, and the extracts are combined Afterwards, the water pump depressurized distillation to recover ethyl acetate, and the yield of the obtained product was 95%. See Figure 7 , see Figure 8 .
Embodiment 3
[0066]In a 50ml ground-mouth conical flask, first add 10ml of ionic liquid hydroxide [1-butyl-3-methylimidazole], then add 0.1mol benzyl mercaptan to the conical flask, mix thoroughly and add 0.12mol Vinyl methacrylate at 1kg / cm 2 Pressure, 25°C constant temperature shaking incubator, shake at a speed of 200 rev. / min; TLC monitors the reaction process, and it shows that the reaction is complete after 2 minutes. After the end, add 60ml of ethyl acetate to the reaction solution for extraction 5 times, and the extracts are combined Afterwards, the water pump depressurized distillation to recover ethyl acetate, and the yield of the obtained product was 91%. See Figure 9 , see Figure 10 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com