Preparation method of atracurium besilate intermediates
A technology for atracurium besylate and intermediates, which is applied in the field of preparation of atracurium besylate intermediates, can solve the problems of high equipment requirements, high raw material costs, harsh operating conditions, and high labor intensity. Achieve the effect of being convenient for industrialized production, low cost and high product purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
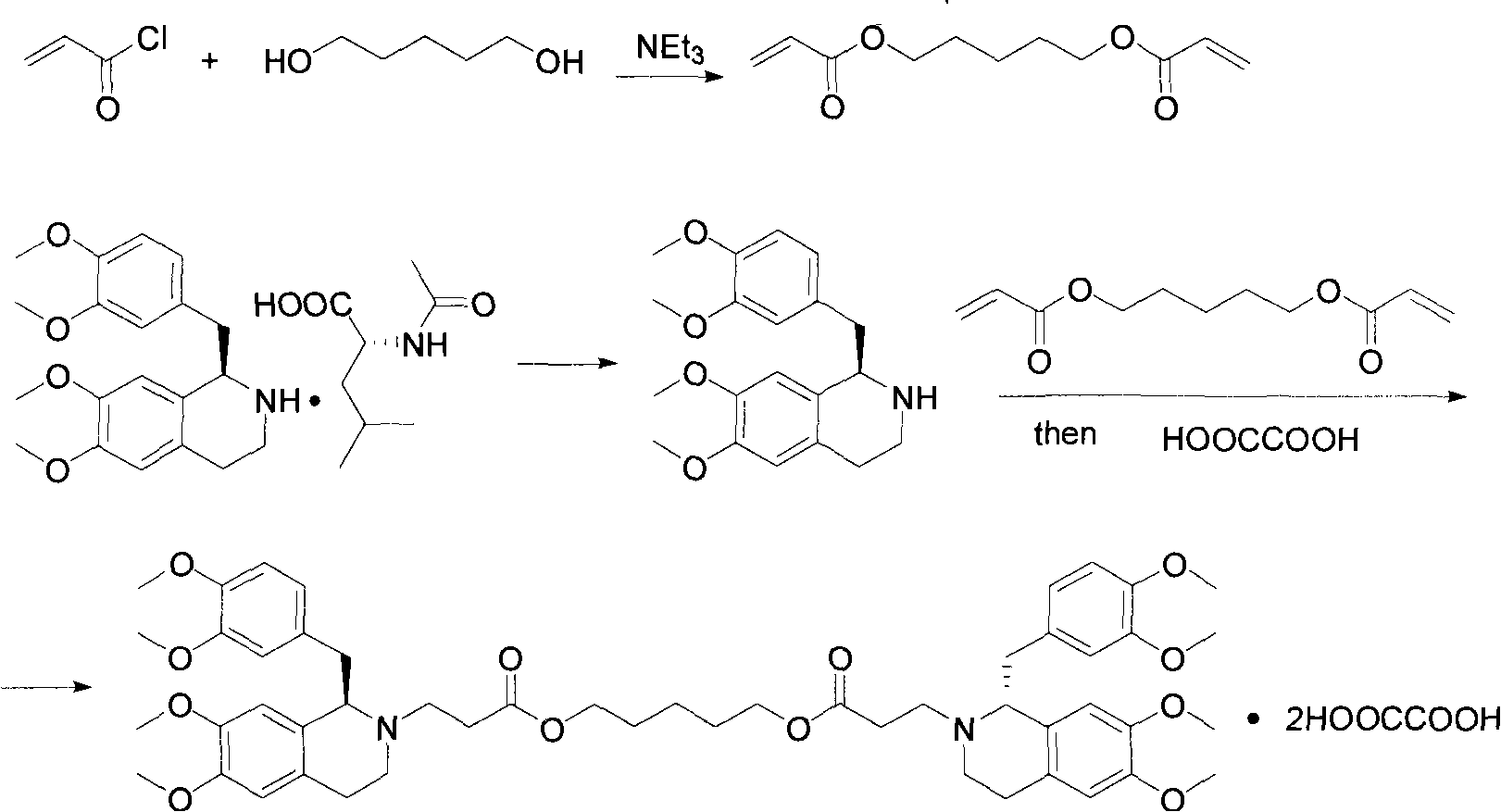
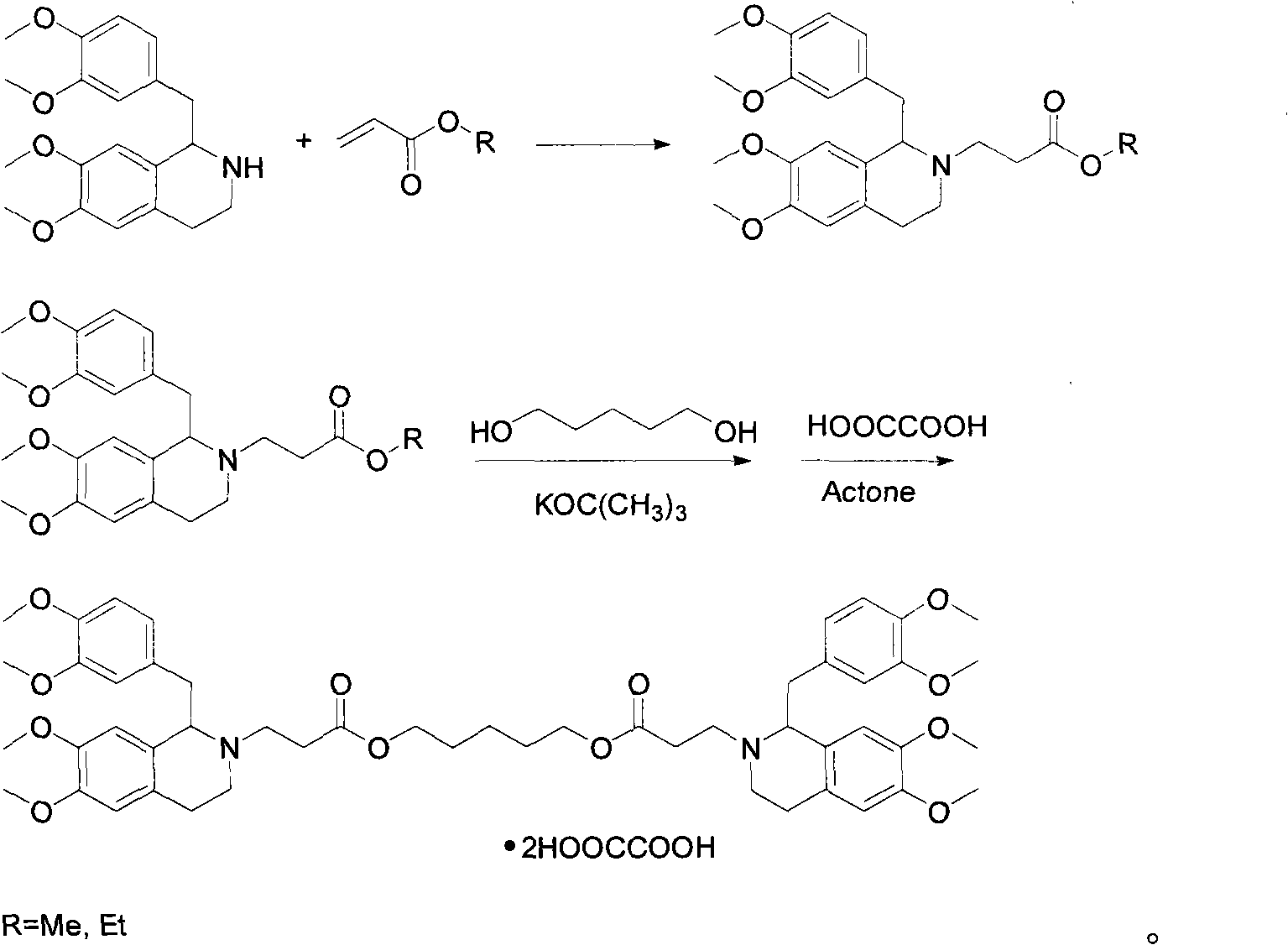
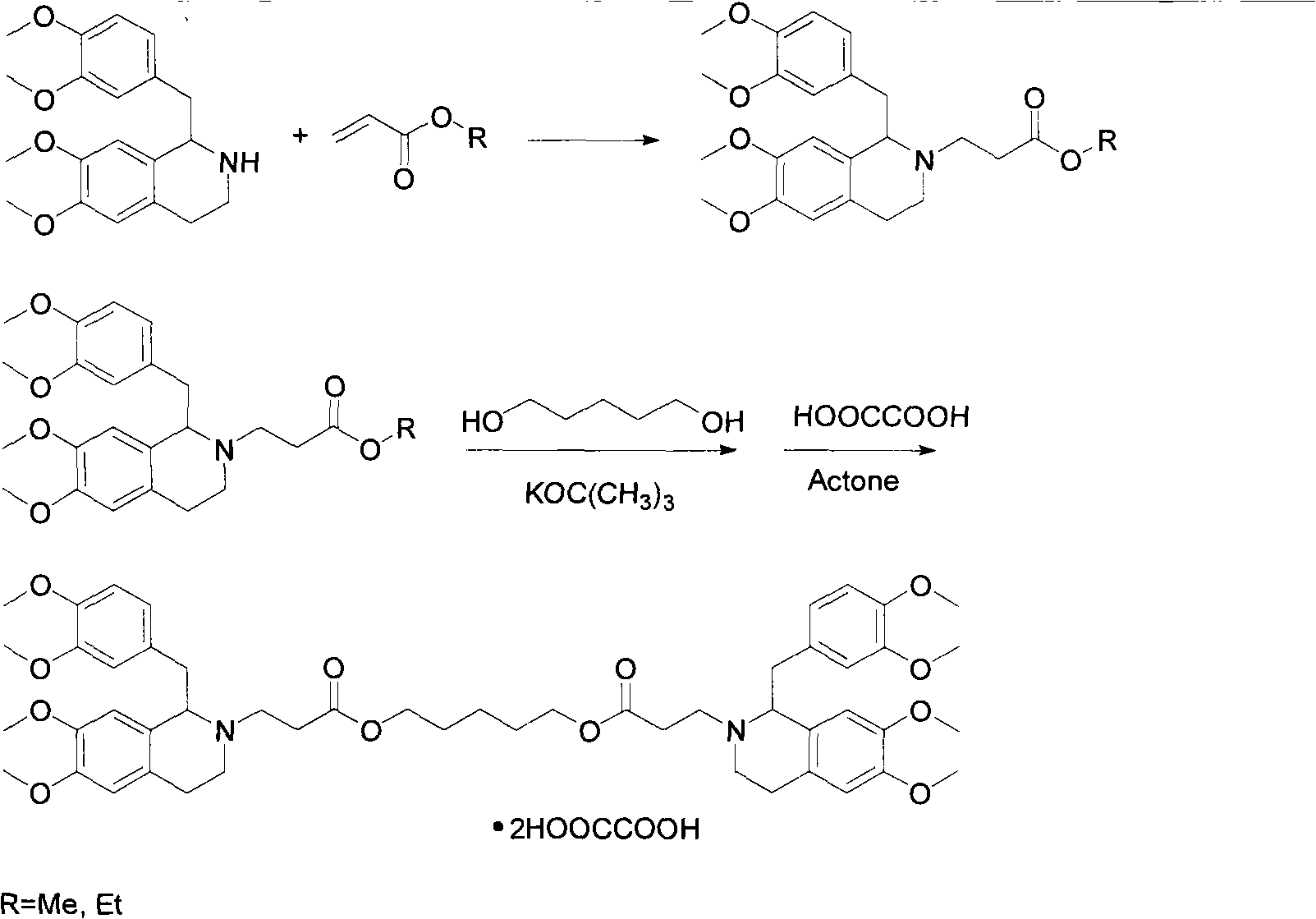
[0015] Dissolve 11.5g of R-tetrahydropapaverine-N-acetyl-L-glutamic acid in an appropriate amount of water, then adjust the pH value to 11 with ammonia water, extract with toluene, and then add 5.8g of methyl acrylate and 70ml of methanol , heated to reflux under stirring conditions for 1.5 hours, then cooled to room temperature, desolvated, filtered, and the filtrate was washed with ether to obtain white solid R-3-[1-(3,4-dimethoxybenzyl) -6,7-dimethoxy-1,2,3,4-tetrahydroisoquinoline]-methyl acrylate 8.9g;
[0016] In the above 3.5g R-3-[1-(3,4-dimethoxybenzyl)-6,7-dimethoxy-1,2,3,4-tetrahydroisoquinoline]-acrylic acid methyl Add 0.43g of pentanediol to the ester, then add 0.05g of potassium tert-butoxide and 100ml of toluene, heat to reflux for 3 hours under stirring conditions, and distill off the azeotrope of methanol and toluene at the same time, then cool to room temperature, wash with water, and the organic phase Drying, desolventization, a yellow viscous substance was...
Embodiment 2
[0018] Dissolve 12.0g of S-tetrahydropapaverine-N-acetyl-L-glutamic acid with an appropriate amount of water, then adjust the pH value to 11 with ammonia water, extract with toluene, and then add 6.2g of ethyl acrylate and 70ml of methanol , heated to reflux under stirring conditions for 1.5 hours, then cooled to room temperature, desolvated, filtered, and the filtrate was washed with ether to obtain white solid S-3-[1-(3,4-dimethoxybenzyl) - 9.2 g of ethyl 6,7-dimethoxy-1,2,3,4-tetrahydroisoquinoline]-acrylate.
[0019] In the above 7.2g S-3-[1-(3,4-dimethoxybenzyl)-6,7-dimethoxy-1,2,3,4-tetrahydroisoquinoline]-acrylic acid ethyl Add 0.88g of pentylene glycol to the ester, then add 0.10g of potassium tert-butoxide and 200ml of toluene, heat and reflux for 3 hours under stirring conditions, and distill off the azeotrope of ethanol and toluene at the same time, then cool to room temperature, wash with water, and the organic phase Drying, desolventization, a yellow viscous subs...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com