Synthesis method of herbicide 2, 4-dichlorphenoxyacetic acid
A technology of dichlorophenoxyacetic acid and a synthesis method, which is applied in the directions of oxidative preparation of carboxylic acid, organic chemistry, etc., can solve problems such as increasing solvent loss, reducing product yield, phenol-containing wastewater, etc., and achieves easy operation, little environmental pollution, Product quality effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
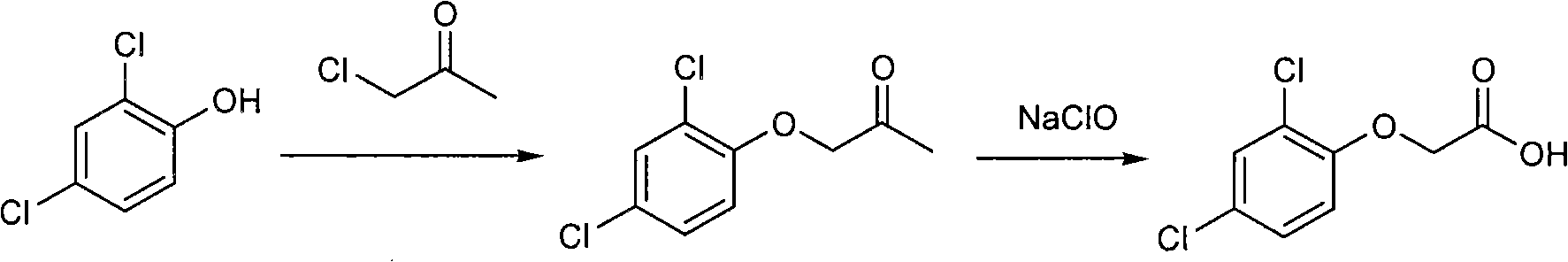
[0026] (1) Synthesis of 2,4-dichlorophenoxyacetone
[0027] In a 1000mL three-necked flask, add 58g (0.55mol) of sodium carbonate, 81g (0.5mol) of 2,4-dichlorophenol, 6.0g of PEG-400, 1.2g of sodium iodide and 200mL of acetonitrile, and heat up to 70°C. A mixture of 55 g (0.6 mol) of monochloroacetone and 50 mL of acetonitrile was slowly added dropwise under vigorous stirring, and the reaction was continued for 3 hours after the drop was completed. After cooling to room temperature, filter, remove the solvent from the filtrate, and collect fractions at 100-102° C. / 1 mmHg by fractional distillation to obtain 101 g of 2,4-dichlorophenoxyacetone with a purity of >99% and a yield of 92%.
[0028] (2) Synthesis of 2,4-dichlorophenoxyacetic acid
[0029] In a 500mL three-necked flask, add 150g of sodium hypochlorite solution with available chlorine ≥ 20%, 20g of sodium hydroxide, add dropwise 22g (0.1mol) of 2,4-dichlorophenoxyacetone and 40mL of 1,4-dioxyl The solution of the hex...
Embodiment 2
[0031] (1) Synthesis of 2,4-dichlorophenoxyacetone
[0032] In a 1000mL three-necked flask, add 65g (0.6mol) of sodium carbonate, 81g (0.5mol) of 2,4-dichlorophenol, 2.5g of tetrabutylammonium chloride, 1.2g of sodium iodide and 200mL of acetone, and heat up to 50°C . A mixture of 55 g (0.6 mol) of monochloroacetone and 50 mL of acetone was slowly added dropwise under vigorous stirring, and the reaction was continued for 3 hours after the drop was completed. After cooling to room temperature, filter, remove the solvent from the filtrate, and obtain 99 g of 2,4-dichlorophenoxyacetone by fractional distillation, with a yield of 90%.
[0033] (2) Synthesis of 2,4-dichlorophenoxyacetic acid
[0034]In a 500mL three-necked flask, add 150g of sodium hypochlorite solution with available chlorine ≥ 20%, 15g of sodium hydroxide, add dropwise a solution of 22g (0.1mol) 2,4-dichlorophenoxyacetone and 40mL of tetrahydrofuran at room temperature with stirring, and dropwise Then continue...
Embodiment 3
[0036] (1) Synthesis of 2,4-dichlorophenoxyacetone
[0037] In a 1000mL three-necked flask, add 300mL saturated aqueous sodium carbonate solution, 81g (0.5mol) 2,4-dichlorophenol, 2.5g tetrabutylammonium chloride, 1.2g sodium iodide and 200mL toluene, heat to 60°C, and A solution of 64 g (0.7 mol) of monochloroacetone and 80 mL of benzene was added dropwise with stirring, and the reaction was continued for 5 hours after the drop was completed. The reaction mixture was lowered to room temperature, and the water layer was separated after standing still. The organic layer was washed with 100 mL of 10% dilute hydrochloric acid solution and water (100 mL×2) respectively. After drying with anhydrous sodium sulfate to remove benzene, fractional distillation gave 95 g of 2,4-dichlorophenoxyacetone with a yield of 88%.
[0038] (2) Synthesis of 2,4-dichlorophenoxyacetic acid
[0039] In a 500mL three-necked flask, add 180g of sodium hypochlorite solution with available chlorine ≥ 20%...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com