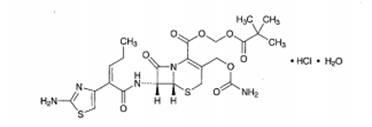
Crystallization method of cefcapene ester hydrochloride monohydrate
A technology for cefcapine hydrochloride and monohydrate, which is applied in the field of crystallization of cefcapine hydrochloride monohydrate, and achieves the effects of being beneficial to drying, beneficial to solvent recovery and good clarity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] 1. Cool 5ml of methanol to below 10°C (-5°C to 10°C), and add 1g of cefcapene hydrochloride to dissolve it.
[0028] 3. Add 0.05g of activated carbon to decarbonize for 20-30 minutes, filter, and wash with a small amount of methanol solution; the obtained solution is solution 1.
[0029] 4. Put 7ml of deionized water and 0.5ml of 30% hydrochloric acid into another reaction kettle and mix. The solution is solution 2, and the temperature is controlled at 20-30°C.
[0030] 6. Add solution 1 dropwise to solution 2 to crystallize (you can also add solution 2 to solution 1 to crystallize). During the dropping process, ensure that the temperature of solution 1 is below 10°C (-5 to 10°C).
[0031] 7. After the dropwise addition, keep stirring at the above temperature for 1 hour, and then cool the temperature to below 10°C.
[0032] 8. Stir at the same temperature (below 10°C) for 1 hour and filter the resulting crystals.
[0033] 9. The crystallization filter cake was washed ...
Embodiment 2
[0036] 1. Cool the mixture of 50ml of methanol and 50ml of acetone to below 10°C (-5°C to 10°C), and add 10g of cefcapene hydrochloride to dissolve it.
[0037] 3. Add 1g of activated carbon to decarbonize for 20-30 minutes, filter, and wash with a small amount of methanol solution; the obtained solution is solution 1.
[0038] 4. Put 80ml of deionized water and 10ml of 36% hydrochloric acid into another reaction kettle to mix. The solution is solution 2, and the temperature is controlled at 5-20°C.
[0039] 6. Add solution 1 dropwise to solution 2 to crystallize (you can also add solution 2 to solution 1 to crystallize). During the dropping process, ensure that the temperature of solution 1 is below 10°C (-5 to 10°C).
[0040] 7. After the dropwise addition, keep stirring at the above temperature for 2 hours, and then cool the temperature to below 10°C.
[0041] 8. Stir at the same temperature (below 10°C) for 2 hours and then filter the resulting crystals.
[0042] 9. The ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com