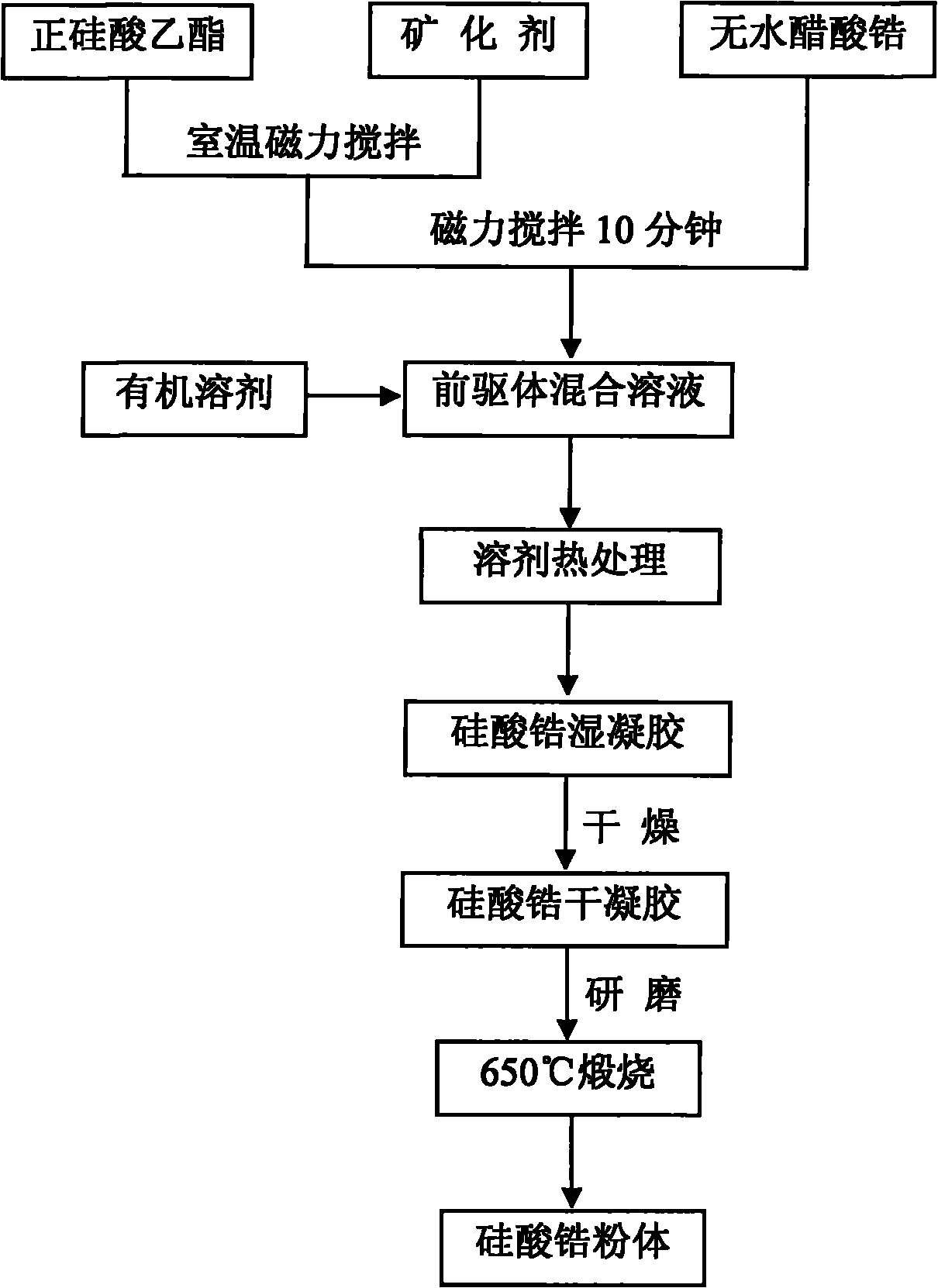
Method for low-temperature synthesis of zirconium silicate powder through non-hydrolytic sol-gel reaction by using zirconium acetate as zirconium source
A non-hydrolysis, zirconium silicate technology, applied in chemical instruments and methods, silicon compounds, non-metallic elements, etc., can solve problems such as unsatisfactory crystal shape development, high synthesis temperature, obvious agglomeration of zirconium silicate powder, etc., to achieve Perfect crystal development, lower synthesis temperature, and less agglomeration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Si(OC 2 h 5 ) 4 (chemically pure), anhydrous zirconium acetate (chemically pure) as the precursor, LiF (chemically pure) as the mineralizer, dichloromethane as the solvent (CH 2 Cl 2 , chemically pure), 0.036molSi(OC 2 h 5 ) 4 After uniform mixing with 0.008mol LiF at room temperature, add 0.03mol anhydrous zirconium acetate, stir on a magnetic stirrer for 15 minutes to obtain a precursor sol, add 35ml of dichloromethane solvent, stir magnetically for 10 minutes, and then pour into 100ml of the liner to form polytetrafluoroethylene In the pressure bomb of vinyl fluoride, the pressure bomb was placed in a constant temperature oven at 110°C to react for 24 hours. After the reaction was completed, it was naturally cooled to room temperature to obtain a white zirconium silicate solid wet gel. Dry the zirconium silicate solid wet gel in a constant temperature oven at 60°C for 6 hours to obtain off-white zirconium silicate xerogel, and zirconium silicate xerogel is pulv...
Embodiment 2
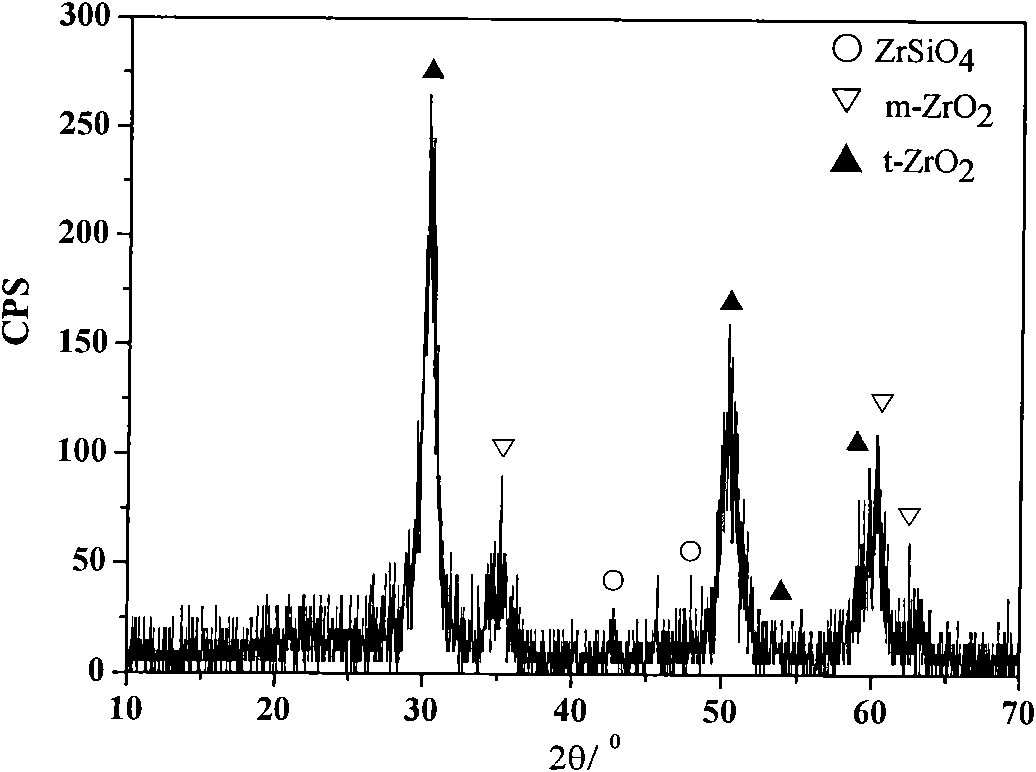
[0026] With 0.036mol Si(OC 2 h 5 ) 4 (chemically pure), 0.026mol anhydrous zirconium acetate (chemically pure) is the precursor, 0.005mol LiF (chemically pure) is the mineralizer, add 38ml ethanol (CH 3 CH 2 OH, chemically pure) as the solvent, zirconium silicate xerogel was incubated at 650°C for 20min, and other technological processes were the same as in Example 1, and the synthetic zirconium silicate powder was shown in the attached Figure 4 .
Embodiment 3
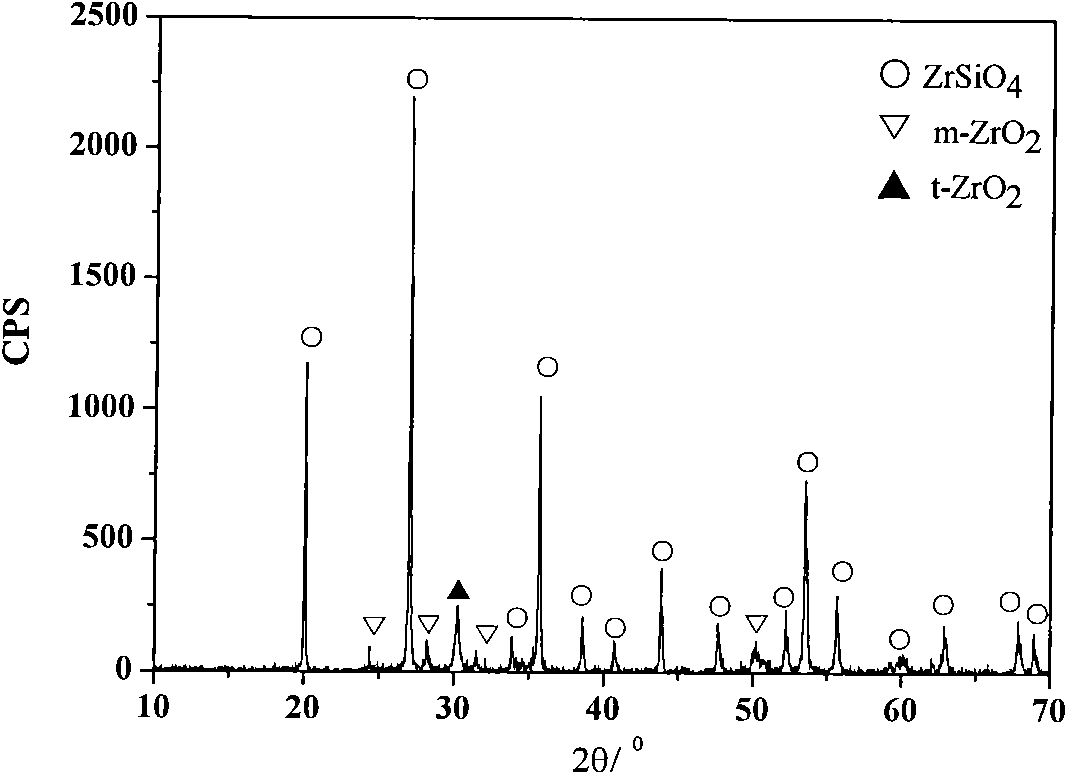
[0028] With 0.036mol Si(OC 2 h 5 ) 4 (chemically pure), 0.028mol anhydrous zirconium acetate (chemically pure) is the precursor, 0.009mol LiF (chemically pure) is the mineralizer, and 50ml of n-butanol (CH 3 CH 2 CH 2 CH 2 OH, chemically pure) is the solvent, the reaction temperature of the pressure bomb is 130 ℃, and the reaction time is 36h. Other technological processes are the same as in Example 1. The synthetic zirconium silicate powder is shown in the appendix Figure 5 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com