Antibody, and coding gene and application thereof
A technology that encodes genes and antibodies, applied in applications, antibodies, gene therapy, etc., can solve problems such as mouse glycosylation patterns, decreased affinity of antigen-antibody, prolong antibody half-life, etc., achieve broad application prospects, combined with the effect of activity inhibition
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Example 1. Acquisition of genes encoding light chain and heavy chain variable regions of antibodies
[0036] According to computer simulation, using the amino acid sequence of the mouse-human chimeric antibody cetuximab as a template, the mouse FR surface gene was humanized to design and synthesize the light chain amino acid sequence L1 and the heavy chain variable region amino acid sequence H1 ;
[0037] Heavy chain variable region H1: the amino acid sequence is shown in sequence 1 in the sequence listing; the coding gene sequence is shown in sequence 4 in the sequence listing;
[0038] Heavy chain variable region H2: the amino acid sequence is shown in sequence 2 in the sequence listing; the coding gene sequence is shown in sequence 5 in the sequence listing;
[0039] Light chain L1: the amino acid sequence is shown in sequence 3 in the sequence listing; the coding gene sequence is shown in sequence 6 in the sequence listing;
[0040] The antibody of the present inv...
Embodiment 2
[0051] Embodiment 2, the preparation of antibody
[0052] 1. Construction of recombinant expression vectors:
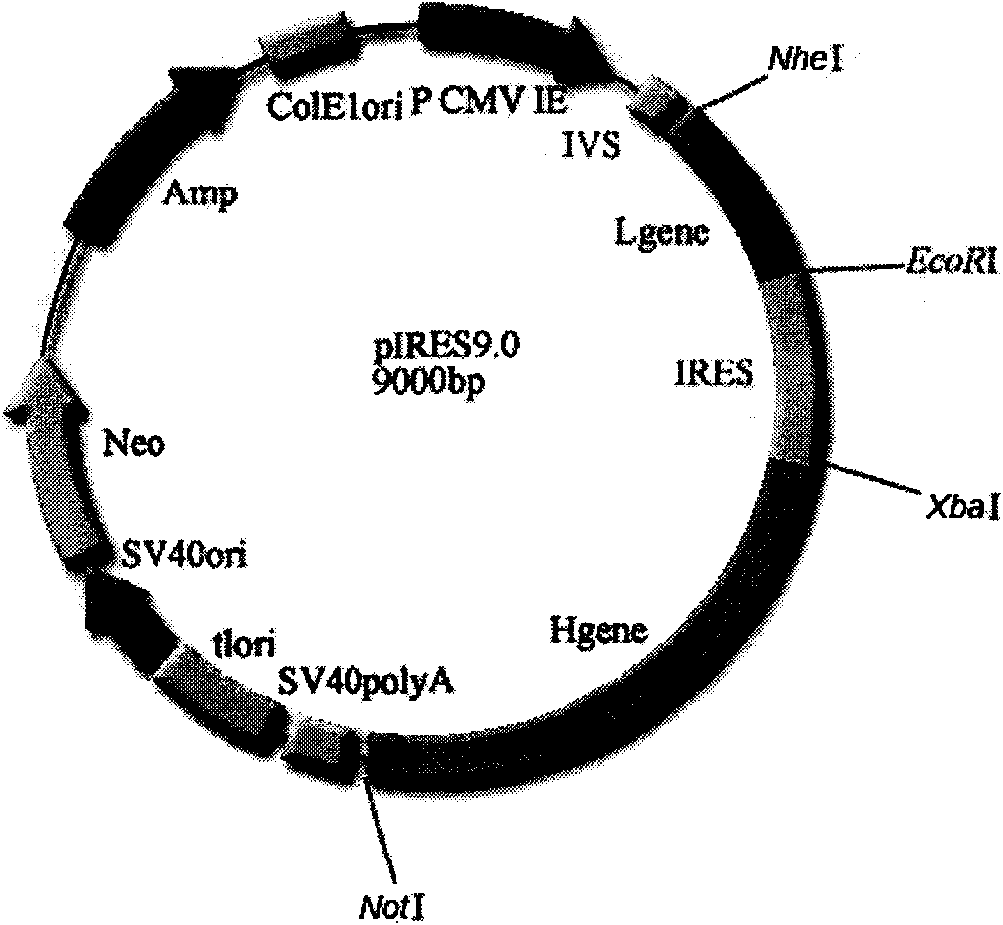
[0053] The pIRES double expression vector was purchased from Clontech Company, the product catalog number is 631605; the pMD18-T expression vector was purchased from Takara Bio Company , the product catalog number is: D504 CA. The pIRES vector itself contains the heavy chain constant region gene.
[0054] Recombinant vector pMD18-T / L1 and pIRES double expression vector were respectively digested with corresponding restriction endonucleases (Nhe I and EcoRI), and after agarose gel electrophoresis, the target fragment was recovered and purified; the light chain gene fragment L1 was combined with The carrier fragments were mixed well, and reacted at 16° C. for 12 h under the action of the ligation reagent. Escherichia coli DH5a was transformed, the clone was picked, the plasmid was extracted and identified by sequencing. The results showed that the insertion direction...
Embodiment 3
[0090] Example 3, Functional Detection of Antibodies
[0091] EGFR protein was purchased from (Sigma), catalog number (E2645-500UN).
[0092] 1. Biacore detects the binding ability of antibody and antigen
[0093] The affinity of antibody C2 to EGFR was determined with Biacore3000 equipment. Prepare 10mmol / L NaAc diluted EGFR protein with different pH values (4.0, 4.5, 5.0 and 5.5), pre-concentrate on the CM5 chip, and select the NaAc diluted protein with the optimal pH value. The purified antibody (i.e. the eluate obtained in step 2 in Example 2) was covalently coupled to the CM5 sensor chip, the mobile phase was HBS-EP (pH7.4), the flow rate was 20 μl / min, and five concentrations were taken Detection of binding affinity of antibody C2 (0, 10.55, 21.1, 42.2 and 84.4nmol / L) to EGFR protein. Affinity was calculated with Biacore3000 accompanying software. At the same time, cetuximab was used as a control.
[0094] The experiment was repeated 3 times, and the results were ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| affinity | aaaaa | aaaaa |
| affinity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com