Compound preparation for treating relevant vascular diseases and preparation method thereof
The technology of compound preparation and compound tablet, which is applied in the field of medicine, can solve the problems of inability to effectively improve the symptoms of intermittent claudication in patients with peripheral arterial occlusion, and achieve the effects of significant vascular-related diseases, simple production process and long shelf life.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
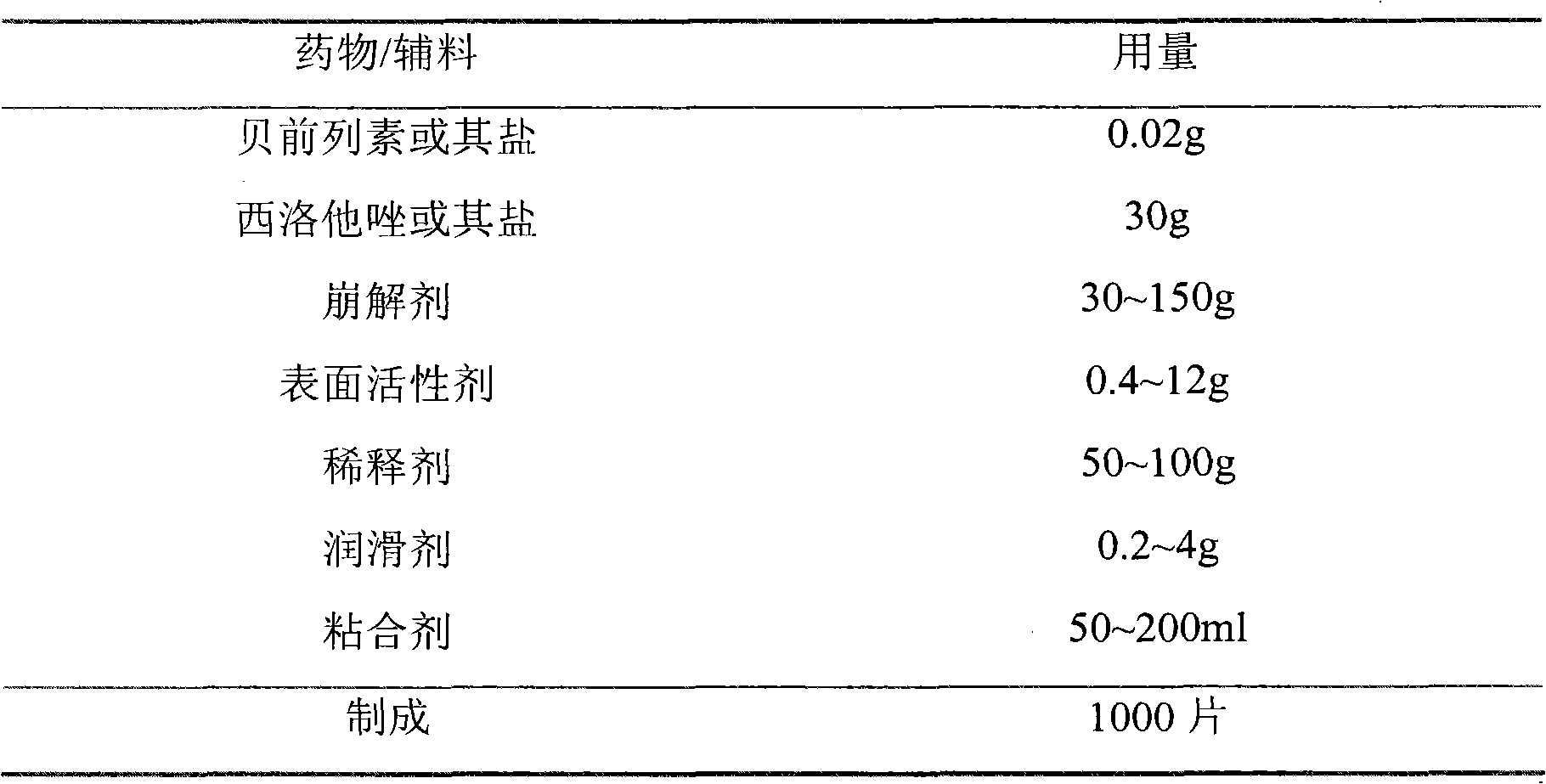
[0075] The preparation of embodiment 1 compound tablet
[0076] formula:
[0077]
[0078]
[0079] Preparation:
[0080] (1) Mix the prescribed amount of beraprost sodium, cilostazol, cross-linked polyvinylpyrrolidone, and sodium lauryl sulfate evenly, and grind in a grinder to obtain a solid dispersion powder with a particle size of 50 μm or less ;
[0081] (2) Add the prescribed amount of starch, make a soft material with 5% polyvinylpyrrolidone and 50% ethanol aqueous solution, pass through a 20-mesh sieve, and ventilate and dry at 35°C;
[0082] (3) After drying, add the prescribed amount of magnesium stearate, mix evenly, granulate, compress into tablets, and obtain compound tablets.
Embodiment 2
[0083] The preparation of embodiment 2 slow-release compound tablet
[0084] formula:
[0085]
[0086] Preparation:
[0087] (1) After mixing evenly the beraprost sodium and cilostazol of the prescribed amount with cross-linked polyvinylpyrrolidone, sodium lauryl sulfate and sodium hydroxypropyl cellulose, they are placed in a grinder and ground to obtain a particle size It is a solid dispersed powder below 50 μm;
[0088] (2) Add the prescribed amount of starch, make a soft material with 5% polyvinylpyrrolidone and 50% ethanol aqueous solution, pass through a 20-mesh sieve, and ventilate and dry at 35°C;
[0089] (3) After drying, add the prescribed amount of magnesium stearate, mix evenly, granulate, compress into tablets, and obtain compound tablets.
Embodiment 3
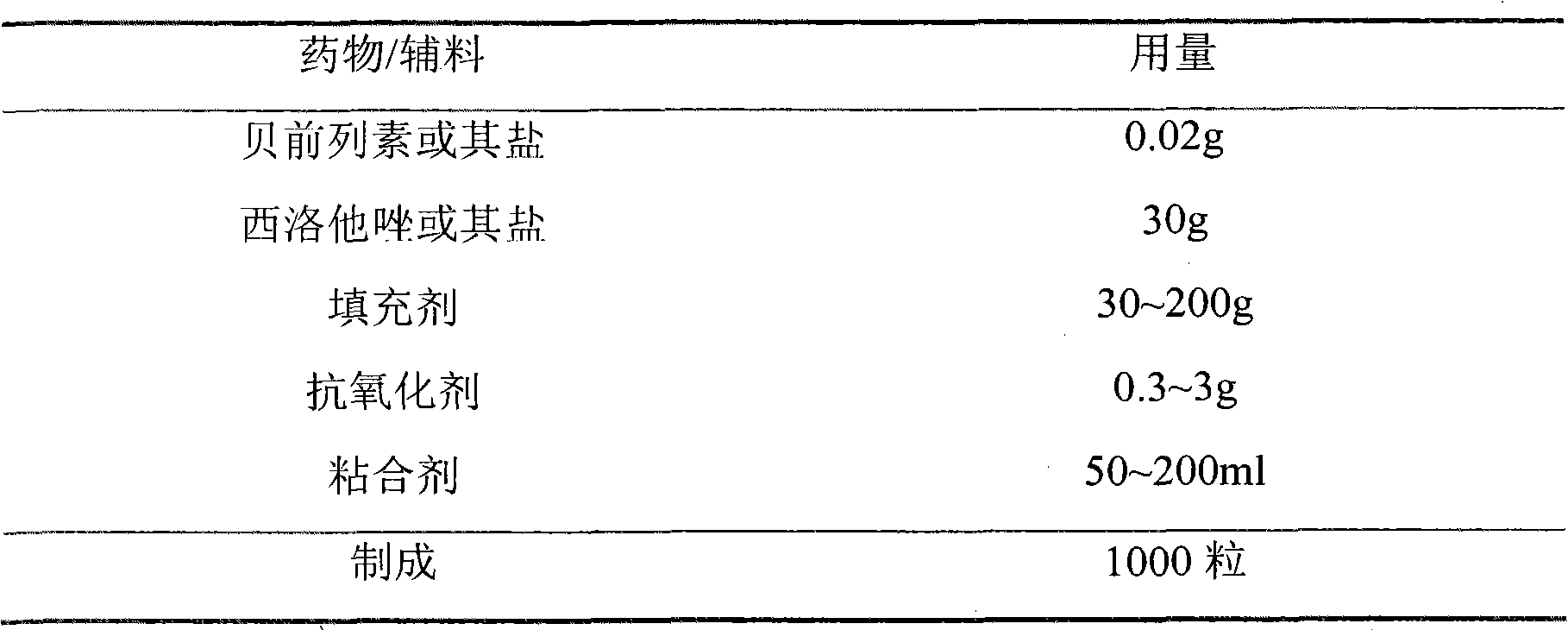
[0090] The preparation of embodiment 3 compound capsules
[0091] formula:
[0092]
[0093] Preparation:
[0094] Take the prescribed amount of beraprost sodium and cilostazol and gradually dilute them with the prescribed amount of starch in equal increments, add the prescribed amount of antioxidant vitamin C and mix evenly, add an appropriate amount of 10% starch slurry, granulate, dry, Whole grains, and then choose moisture-proof and anti-oxidation coating powder to coat it, dry, sieve, and pack into capsules.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com