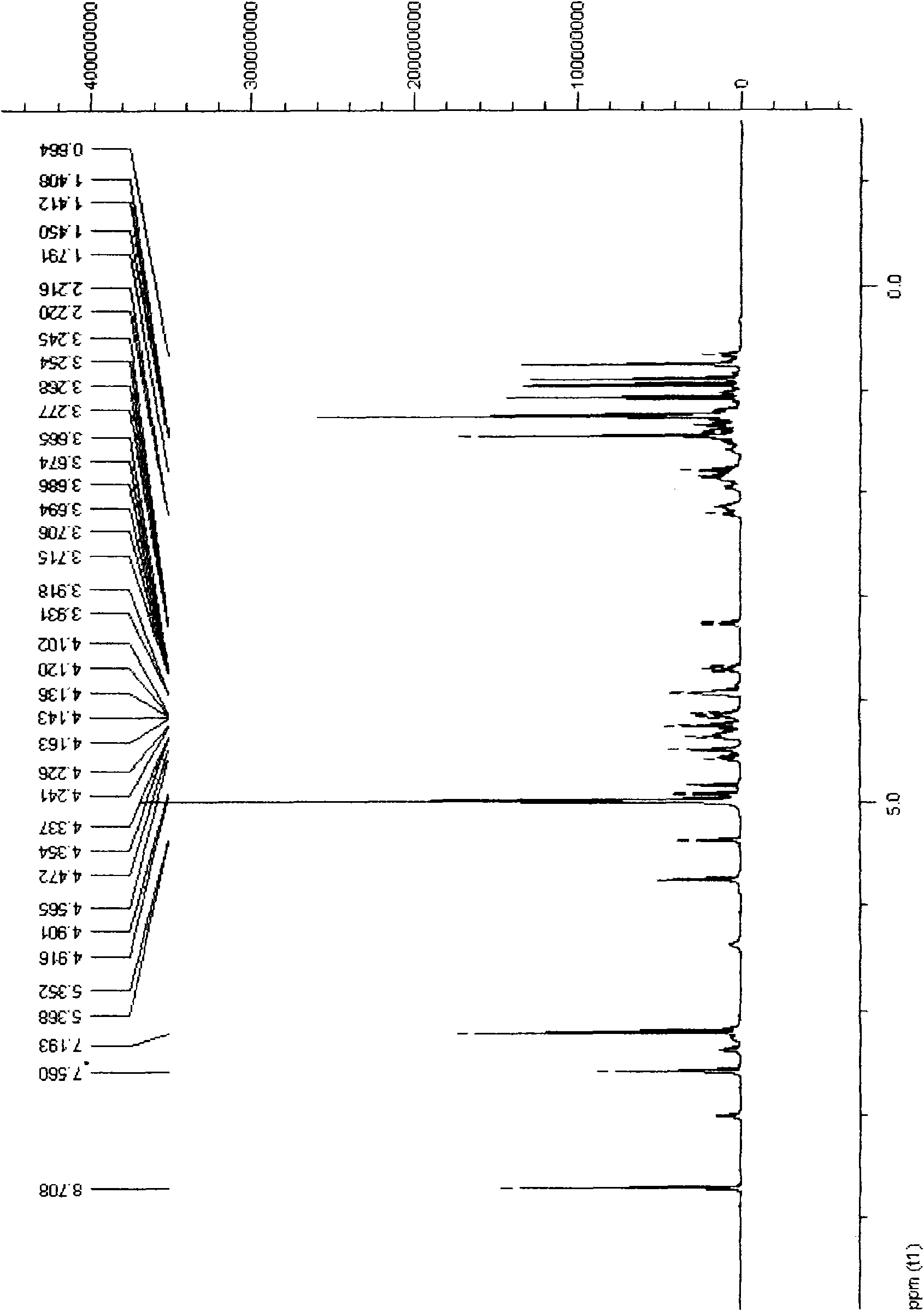
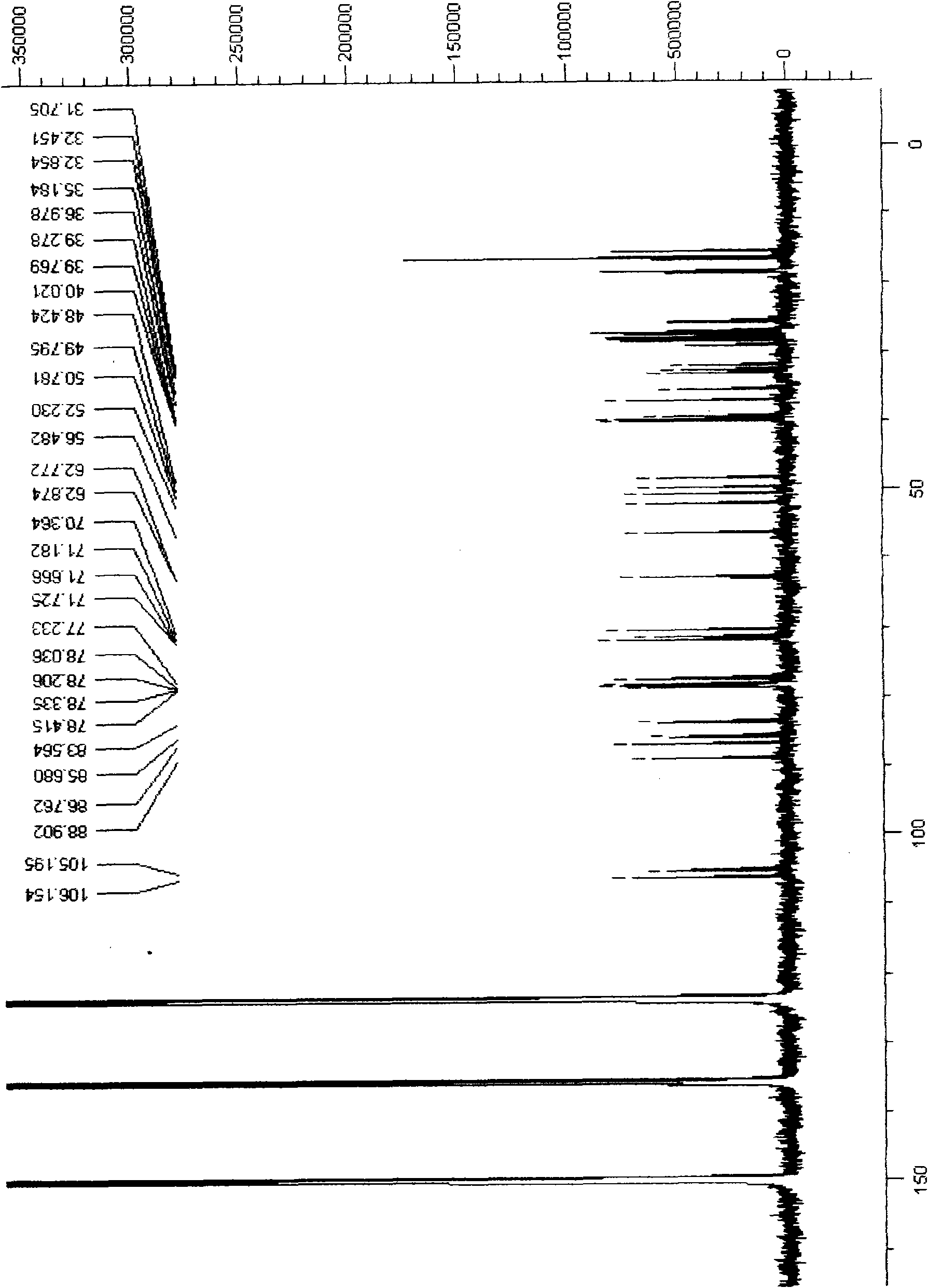
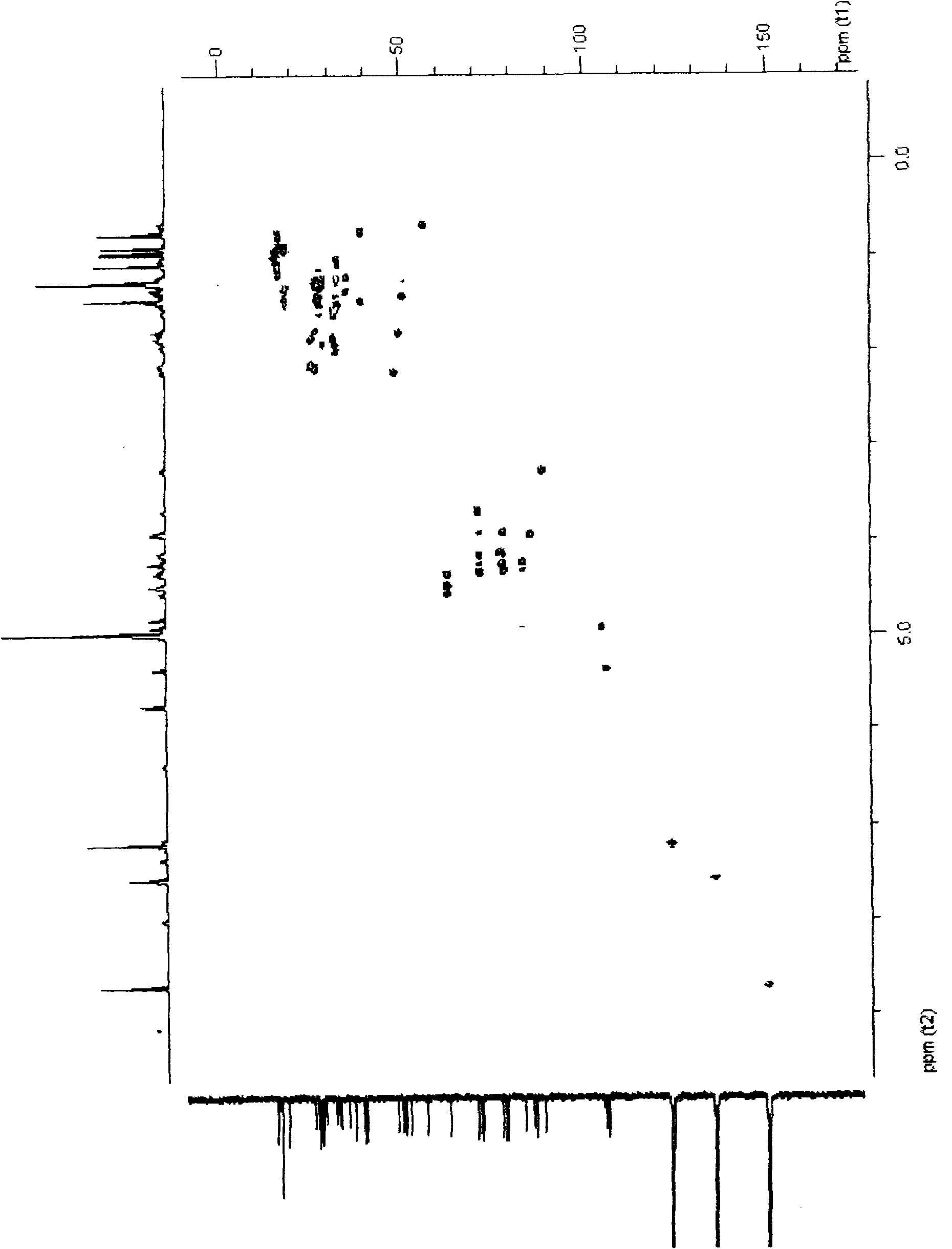
24(R)-pseudoginsenoside-GQ as well as semisynthesis method and medicinal application thereof
A technology of pseudoginsenosides and ginsenosides, which is applied in the field of 24-pseudoginsenoside-GQ and its semi-synthesis, can solve the problems that have not been seen, and achieve excellent and significant curative effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
experiment example 1 24
[0029] Experimental example 1. Effect of 24(R)-pseudoginsenoside-GQ on hemorrhagic shock
[0030] 1. Experimental materials and methods
[0031] Materials: 6 beagle dogs; 24(R)-PGQ: 10mg / ml aqueous solution
[0032] Methods: The dog was anesthetized intravenously with pentobarbital sodium (1ml he), was fixed on the operating table in supine position, the trachea was separated, and the trachea was intubated. Separate the common carotid artery and connect it to RM-6000 four-lead physiological recorder to measure: BP (SBP / DBP); separate the right bone artery and measure LVSP, LVEDP, ±dp / dt; separate the right femoral vein for drug use .
[0033]Separate the right-dipping femoral artery, prepare for arterial bloodletting, connect ECG leads, measure ECG to calculate HR, connect force sensor with silk thread at the xiphoid process skin, measure respiratory rate, stabilize for 10 minutes after the operation, record all normal indicators, and then Bleeding through the femoral arter...
experiment example 2 24
[0078] Experimental Example 2. Effect of 24(R)-Pseudoginsenoside-GQ on Hemodynamics of Normal Anesthetized Dogs (n=6)
[0079] 1. Experimental materials and methods
[0080] Materials: 6 Beagle dogs, weighing 13kg to 15kg, 3 males and 3 males, 24(R)-pseudoginsenoside-GQ: 10mg / ml solution;
[0081] Method: The method is the same as Experiment 1, so it is omitted.
[0082] 2. Results
[0083] 1. Effect on lipid peroxide (LPO)
[0084] The content of 120'LPO in the 24(R)-PGQ small and high dose groups after administration was significantly lower than that in the saline control group, and the difference between the two groups was significant (p<0.05).
[0085] The content of 120'LPO in the dopamine group was significantly lower than that in the saline control group after administration, and the difference between the two groups was significant (p<0.05). The results are shown in Table 2-1.
[0086] Table 2-124(R)-PGQ Effect on LPO(A / ml)
[0087]
[0088] p<0.05 vs. 0.9% ...
experiment example 3
[0097] Experimental example three, MTT (tetrazolium salt) method to measure the selection of the killing effect of 24 (R)-pseudoginsenoside-GQ on tumor cells
[0098] (1) Experimental design
[0099] Tumor lines used: MCF-7 human breast cancer cells, PC3M human prostate cancer cells, NCI-H446 human lung cancer cells, Helas human cervical cancer cells, H8 human colon cancer cells, SHG-44 human glioma cells, SK-OV- 3 human ovarian cancer cells, 7 human tumor cell lines.
[0100] (Note: Different cells use different culture medium: RPMI1640, IMDM, DMEM.)
[0101] Test grouping: 24(R)-pseudoginsenoside-GQ dosage group:
[0102] 1.0μg / ml, 2.5μg / ml, 5μg / ml, 7.5μg / ml, 10μg / ml, 12.5μg / ml, 15μg / ml, 17.5μg / ml, 20μg / ml;
[0103]Blank control group: solvent;
[0104] Positive control group: 5-fluorouracil (1.0 μg / ml).
[0105] (2) method:
[0106] ① Preparation of MTT solution: put 250mgMTT into a small beaker, add 50ml of PBS (pH7.4, 0.01M), stir for 30min, prepare a solution with ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com