Benserazide sustained-release microspherical composition and preparation method thereof
A technology for sustained-release microspheres and compositions, which is applied in the directions of pharmaceutical combinations, pharmaceutical formulations, and medical preparations of inactive ingredients, etc., can solve the problems in the method, action and memory of microspheres prepared by benserazide, which are not reported in relevant literature. , inconvenience and other problems, to achieve the effect of good re-dispersibility, avoid the effect of the effect, and improve the therapeutic effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
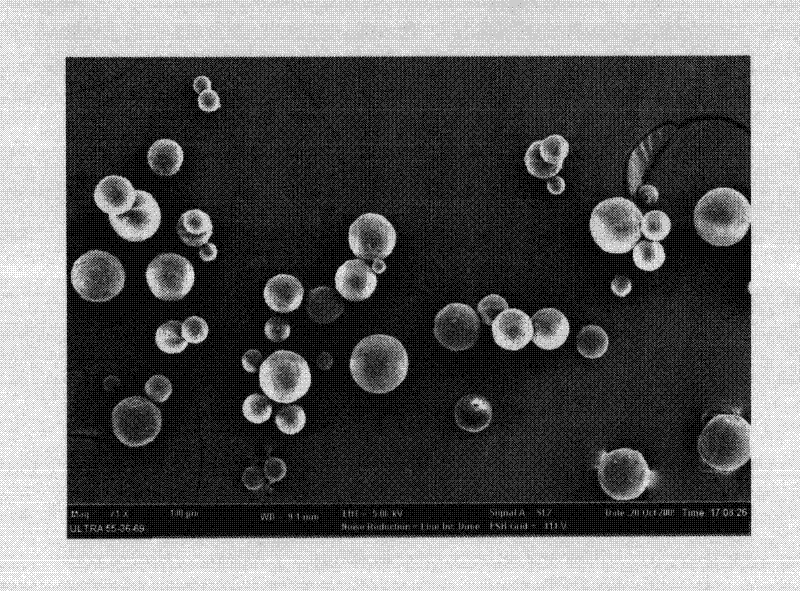
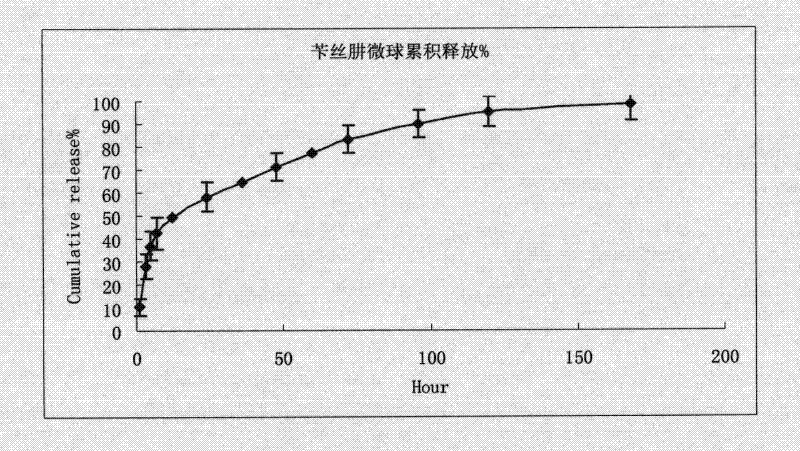
Image
Examples
Embodiment 1
[0029] ①Put 100mg of benserazide into 0.4-10μm with a microscope first, if not, pulverize it into 0.4-5μm with a pulverizer;
[0030] ② Preparation of benserazide sustained-release microsphere composition
[0031] (a) 37.5 mg of polylactic acid (PLA, with a molecular weight of 90,000-140,000) weighed into a dichloromethane organic solution with a concentration of 15% by weight and 12.5 mg of benserazide microparticles weighed in ① were mixed and stirred , vortex or ultrasonic for 1-5 minutes to form a uniform suspension, that is, a solid-in-oil (S / O) emulsion; the theoretical percentage content of benserazide to be prepared is 25% slow-release microspheres.
[0032] (b) adding the emulsion obtained in step (a) to 10 mL of an aqueous solution of 5% sodium chloride and 1% polyethylene glycol (the molecular weight of PVA is 146,000-186,000, alcoholysis degree 98-99%) aqueous solution Stir, vortex or sonicate for 0.1-5 minutes to form a double emulsion;
[0033] (c) adding the d...
Embodiment 2
[0040] ①Preparation of benserazide microparticles
[0041] 100mg benserazide, first observe with a microscope whether it is 0.4-10μm, if not, it can be pulverized into 0.4-5μm with a pulverizer;
[0042] ② Preparation of benserazide sustained-release microsphere composition
[0043] (a) Weigh 495 mg of polyglycolic acid-polylactic acid (PLGA, d, l-lactide: 46-52% Mole and glycolide 48-54% Mole; molecular weight 5000-6000) to prepare a weight percent concentration of 30% The organic solution of dichloromethane and 5 mg of benserazide particles obtained by weighing ① are mixed and stirred, vortexed or ultrasonicated for 1-5 minutes to form a uniform suspension, that is, a solid-in-oil (S / O) emulsion; will be prepared as The theoretical percentage of benserazide is 1% slow-release microspheres.
[0044] (b) adding the emulsion obtained in step (a) to 10 mL of an aqueous solution of polyethylene glycol (the molecular weight of PVA is 146,000-186,000, the degree of alcoholysis 98...
Embodiment 3
[0052] ①Preparation of benserazide microparticles
[0053] 100mg benserazide, first observe with a microscope whether it is 0.4-10μm, if not, it can be pulverized into 0.4-5μm with a pulverizer;
[0054] ② Preparation of benserazide sustained-release microsphere composition
[0055] (a) 37.5 mg of polyglycolic acid-polylactic acid (PLGA, d, l-lactide: 46-52% Mole and glycolide 48-54% Mole; molecular weight 5000-6000) were weighed to prepare a weight percent concentration of 15 % organic solution of dichloromethane and 12.5 mg of benserazide microparticles obtained by weighing ① are mixed and stirred, vortexed or ultrasonicated for 1-5 minutes to form a uniform suspension, that is, a solid-in-oil (S / O) emulsion; The theoretical percentage content of prepared benserazide is 25% slow-release microspheres.
[0056] (b) adding the emulsion obtained in step (a) to 10 mL of an aqueous solution of 5% sodium chloride and 1% polyethylene glycol (the molecular weight of PVA is 146,000-...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com