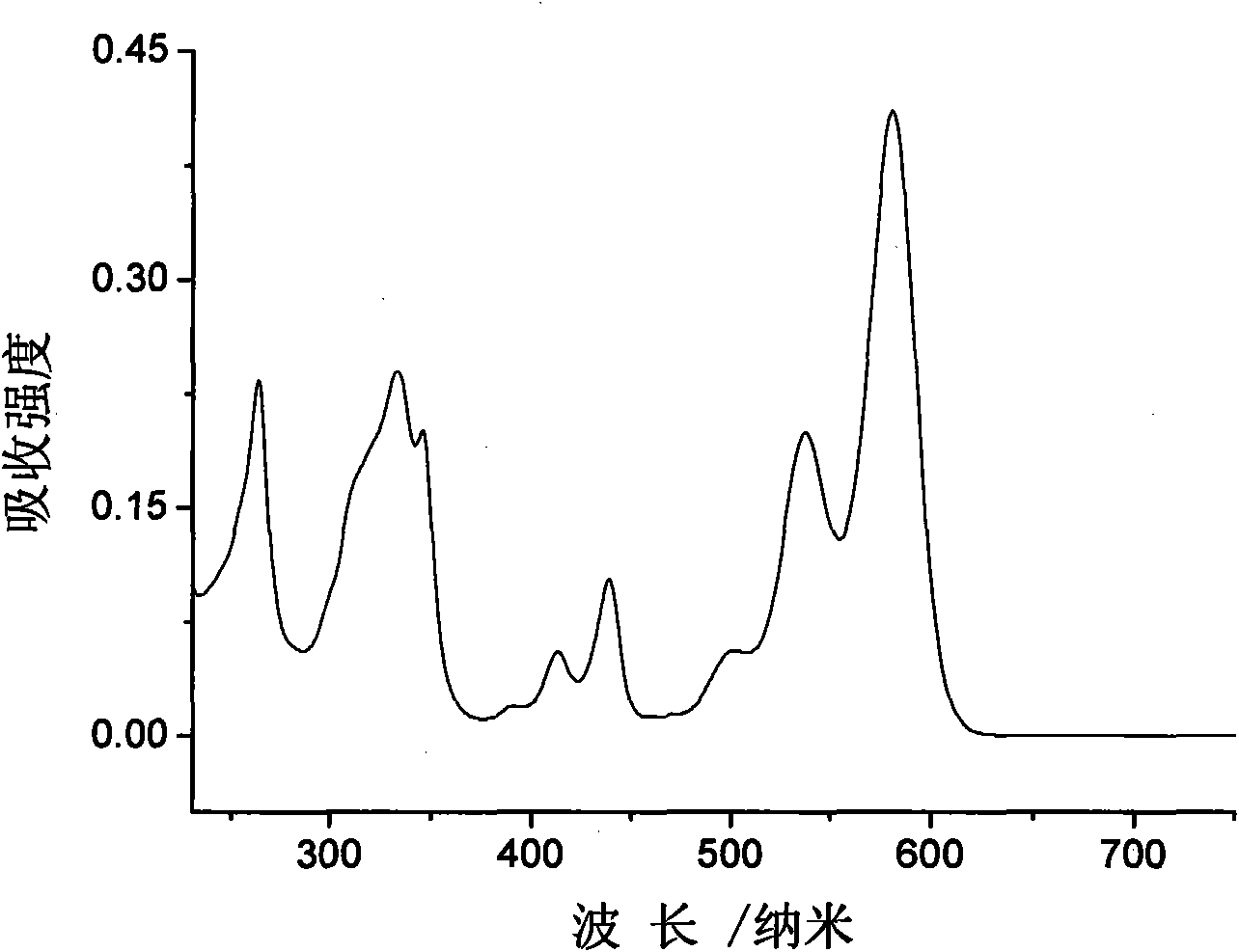
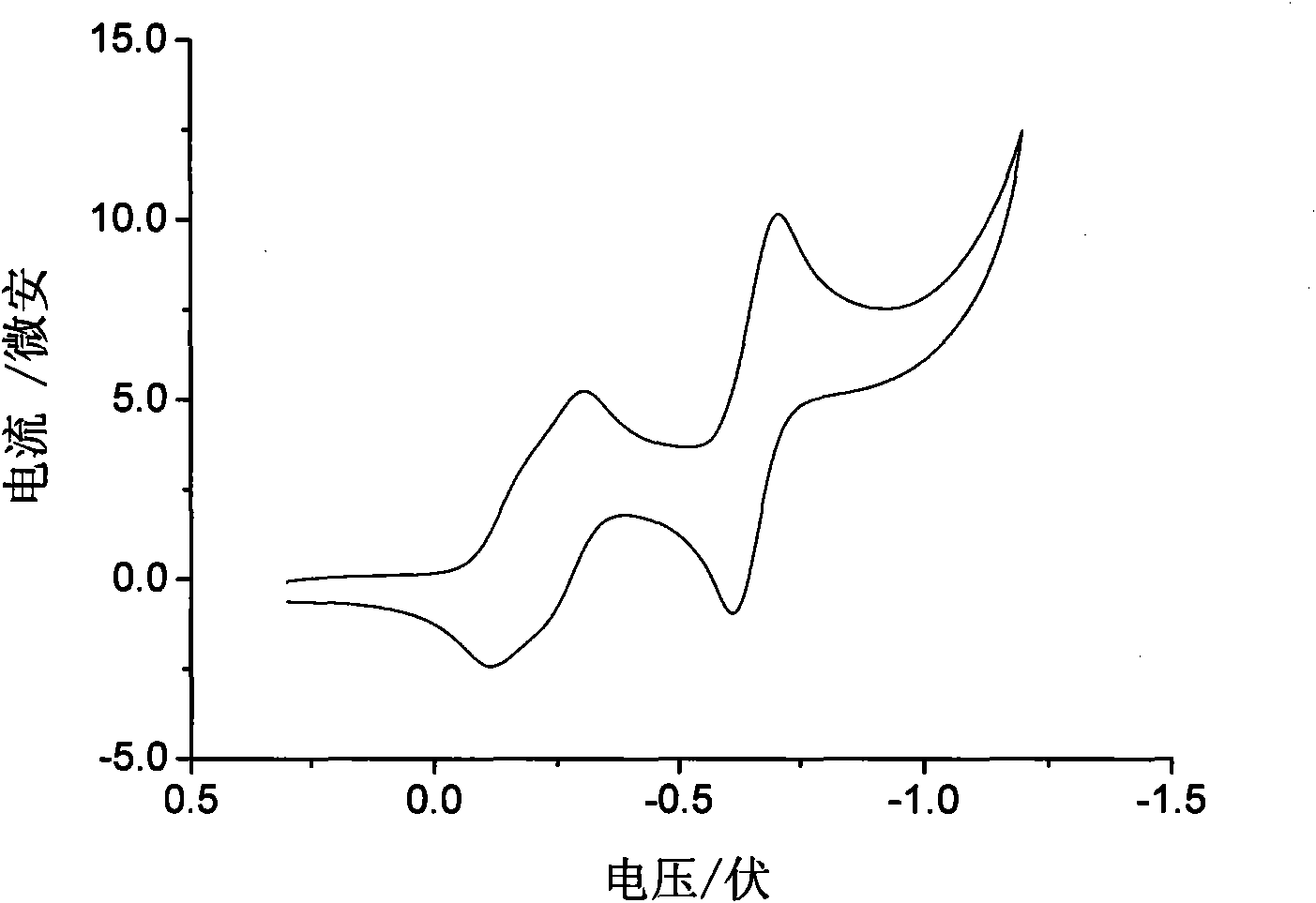
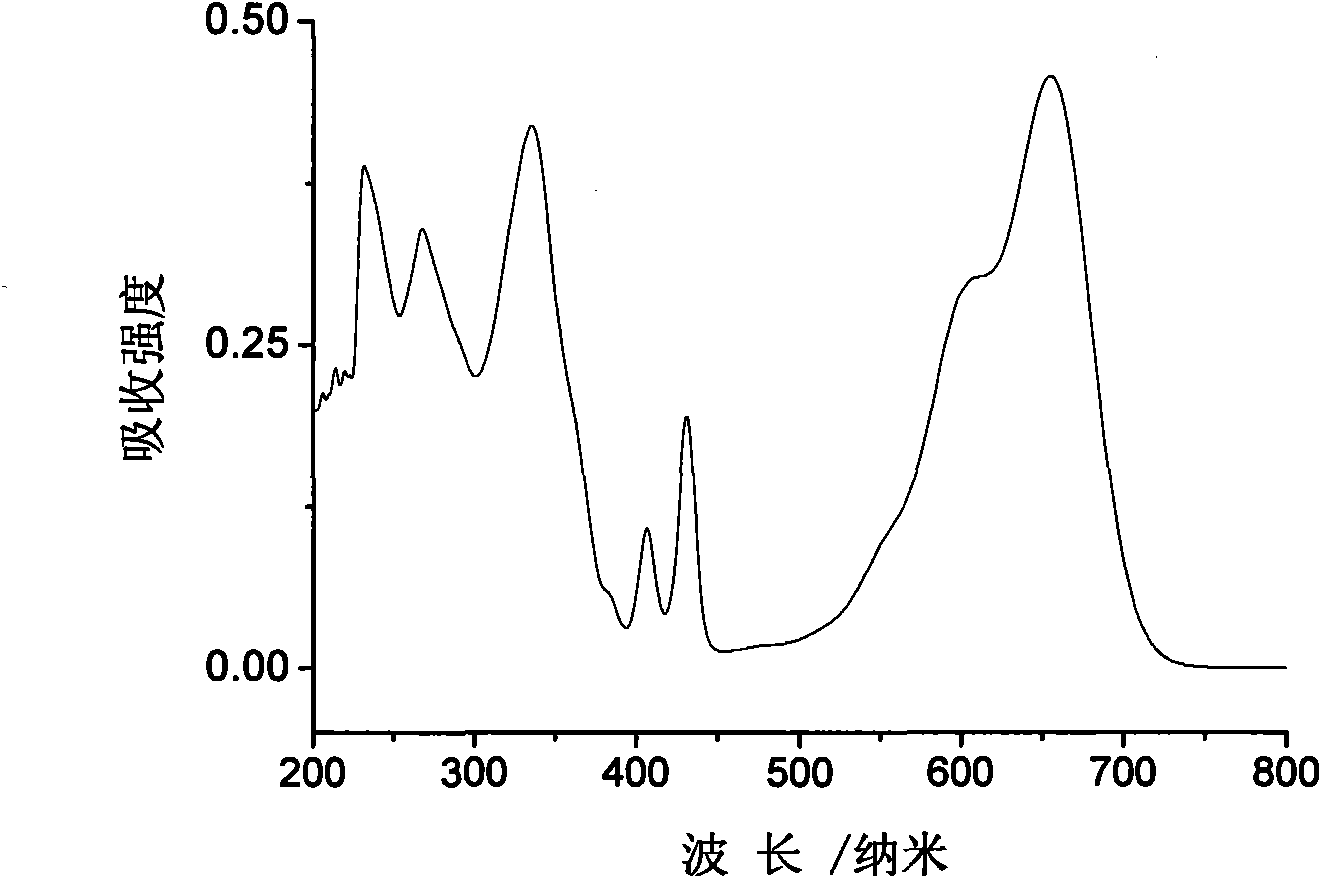
Heterocyclic-sulfur fused naphthalenetetracarboxylic acid diimide derivatives, preparation method and application thereof
A technology of naphthalene tetracarboxylic acid diimide and tetrabromonaphthalene tetracarboxylic acid diimide, which is applied in the field of naphthalene tetracarboxylic acid diimide derivatives, and can solve the problem of difficult formation of π-π stacking and OTFT devices Low electron mobility and other problems, to achieve the effect of low synthesis cost, simple and effective synthesis method, high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0060] Example 1: N, N'-bis(2-octyl-dodecyl)-[2,3-d:6,7-d']-bis[2-cyano-2-(1,3 -Synthesis of ethyl dithiacyclopentene-2-ylidene)acetate]-naphthalene-1,4,5,8-tetracarboxylic diimide (1).
[0061] Concrete synthetic steps are:
[0062] Ethyl cyanoacetate (CNCH 3 COOEt, 297mg, 2.63mmol) and carbon disulfide (CS 2 , 0.16mL, 2.63mmol) was dissolved in 25mL THF, and at 0-5°C, under the protection of nitrogen, slowly added the above solution dropwise to a container containing 132mg (5.3mmol) sodium hydride (NaH, 96%) and 5mL THF In the there-necked flask, after 0.5 hours, the reaction solution was raised to room temperature, and the stirring reaction was continued for 4 hours, and 300 mg (0.26 mmol) of N,N'-bis(2-octyl-dodecyl)- 2,3,6,7-Tetrabromonaphthalene tetracarboxylic acid diimide, at room temperature, continue to react for 0.5 hours, and spin off the solvent under reduced pressure. The crude product was purified by silica gel chromatography (eluent: dichloromethane / petrole...
Embodiment 2
[0066]Example 2: N, N'-bis(2-octyl-dodecyl)-[2,3-d:6,7-d']-bis[2-cyano-2-(1,3 Synthesis of -dithiacyclopentene-2-ylidene)-n-hexyl acetate]-naphthalene-1,4,5,8-tetracarboxylic diimide (2).
[0067] Concrete synthetic steps are:
[0068] Using n-hexyl cyanoacetate instead of ethyl cyanoacetate, the synthesis method was the same as that in Example 1, and a dark brown solid (2) was prepared with a yield of 59%.
[0069] Mass Spectrum: [MS(TOF)]m / z: 1308.7(M + ).
[0070] Elemental analysis: Molecular formula: C 74 h 108 N 4 o 8 S 4 ; Theoretical: C, 67.85; H, 8.31; N, 4.28; Found: C, 67.87; H, 8.57; N, 3.85.
[0071] H NMR spectrum: 1 H-NMR (300MHz, CDCl 3 )δ (ppm): 0.854-0.942 (m, 9H, CH 3 ), 1.223-1.368 (m, 38H, CH 2 ), 1.741-1.832 (m, 2H, CH 2 ), 2.043 (b, 1H, CH), 4.227-4.251 (d, J=7.2Hz, 2H, -CH 2 -N), 4.335-4.378(t, J=6.5Hz, 2H, -CH 2 -O).
Embodiment 3
[0072] Example 3: N, N'-bis(n-octyl)-[2,3-d:6,7-d']-bis[2-cyano-2-(1,3-dithiecyclopentene Synthesis of -2-ylidene)acetate 2-ethylhexyl]-naphthalene-1,4,5,8-tetracarboxylic diimide (3).
[0073] Concrete synthetic steps are:
[0074] Replace ethyl cyanoacetate and N, N'-two (2-octyl-dodecyl)-2,3,6,7-tetrabromonaphthalene tetracarboxylic acid diimide, synthetic method is the same as step in embodiment 1, prepares deep Brown solid (3), 40.8% yield.
[0075] Mass Spectrum: [MS(TOF)]m / z: 1051.1(M + +Na), 1074.1 (M + +2Na).
[0076] Elemental analysis: Molecular formula: C 54 h 68 N 4 o 8 S 4 ; Theoretical: C, 63.01; H, 6.66; N, 5.44; Found: C, 63.03; H, 6.61; N, 5.34.
[0077] H NMR spectrum: 1 H-NMR (300MHz, CDCl 3 )δ (ppm): 0.926-0.932 (m, 9H, CH 3 ), 1.302(b, 18H, CH 2 ), 1.784-1.1.814 (m, 3H, overlapped, (CH 3 )CH 2 (CH) and CH), 4.282-4.295 (m, 4H, overlapped, -CH 2 -N and -CH 2 -O).
[0078] The synthetic route of embodiment 4 and 5 is shown in the followi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Electron mobility | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com