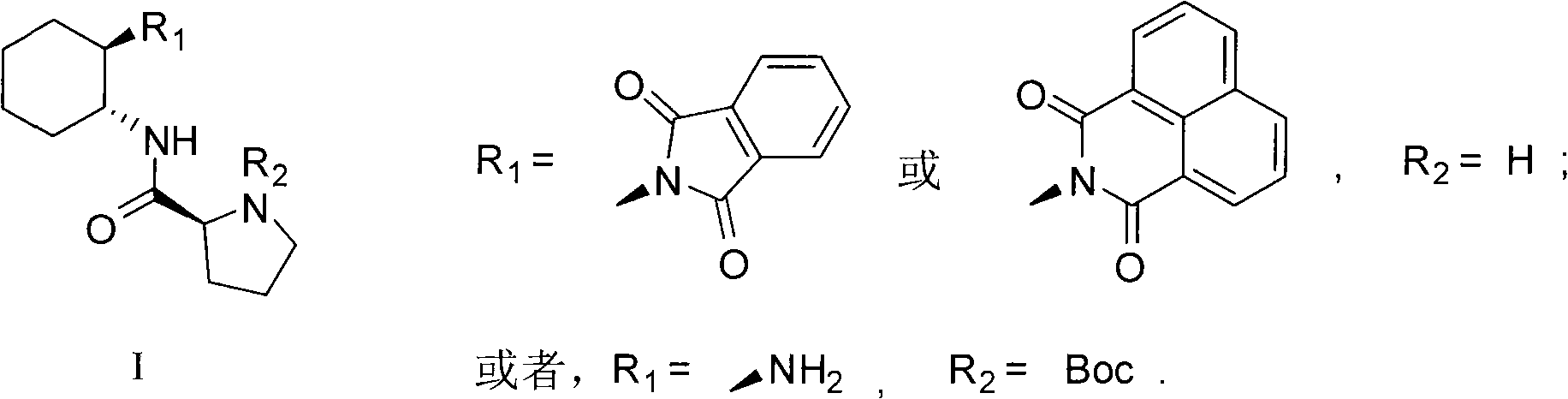
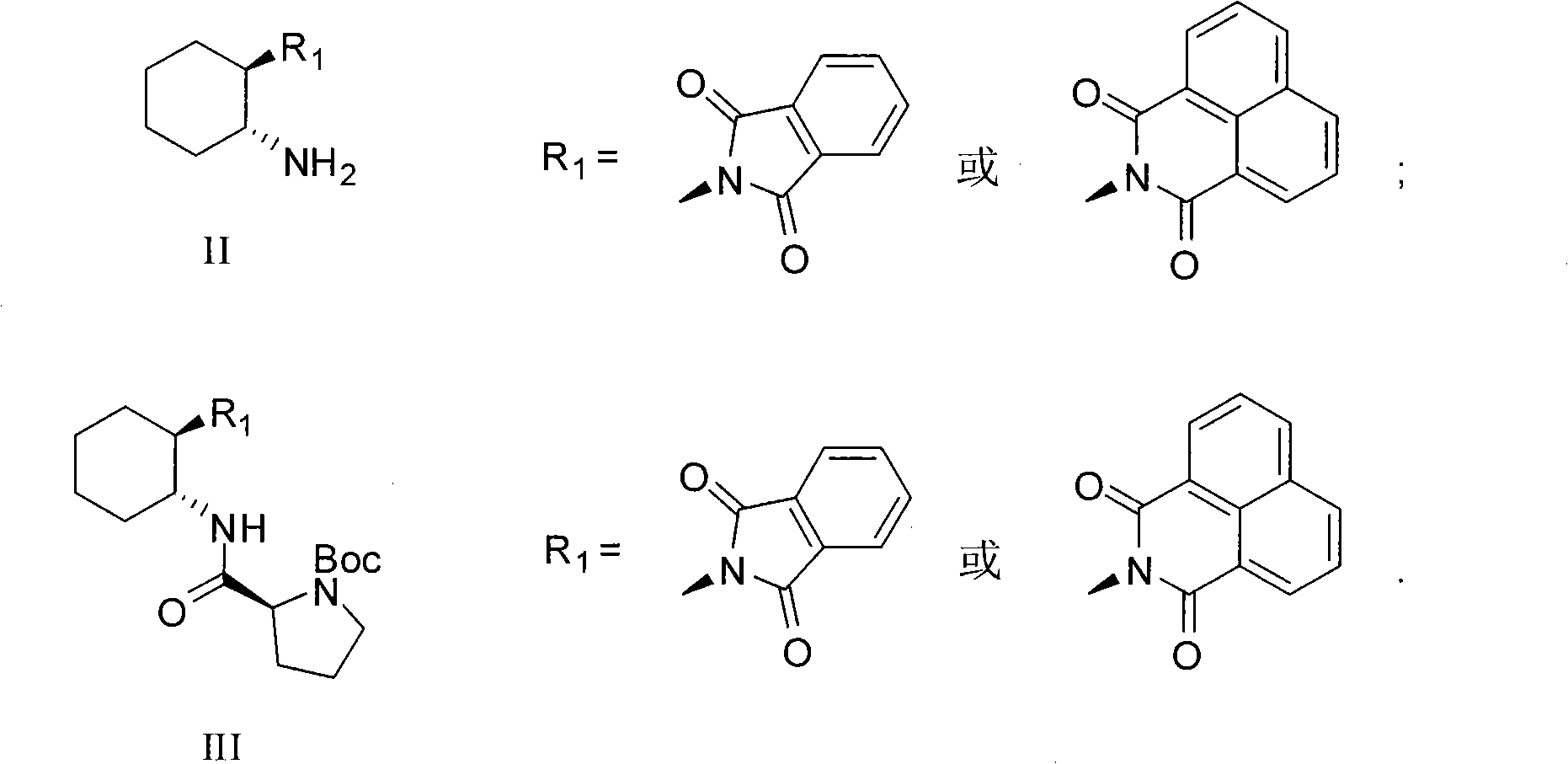
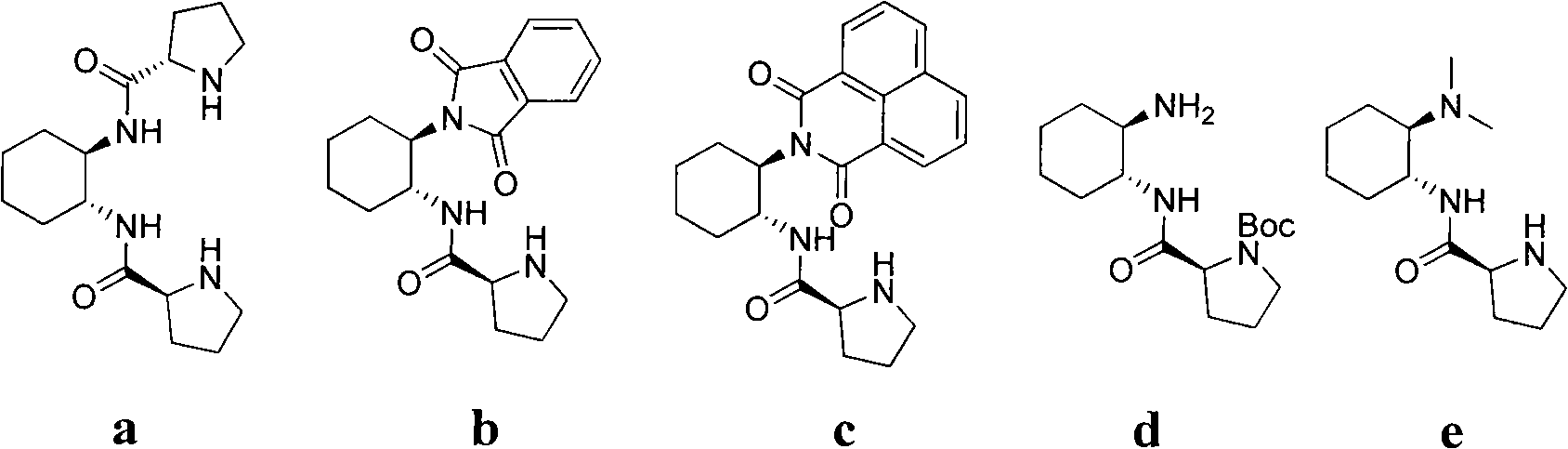
L-prolinamide derivative, preparation method and application of same
A technology of prolineamide and derivatives, applied in the field of chemistry, can solve problems such as cost increase, and achieve the effects of easy operation and high selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] The preparation of embodiment 1, L-prolineamide derivative b
[0025]
[0026] A. The preparation of intermediate g: under argon protection, temperature 0 ℃, add N-Boc-L-pro (4.7g, 22mmol) and triethylamine (2.3g, 22mmol) to anhydrous dichloromethane solution (80mL) was slowly added dropwise ethyl chloroformate (2.6g, 24mmol), after the addition was completed, stirred at 0°C for 30 minutes, then slowly added dropwise anhydrous dichloromethane solution (15mL) of compound f (4.9g, 20mmol), Add and react at room temperature for 4 hours; after the reaction is completed, the reaction mixture is successively washed with potassium bisulfate solution, saturated sodium bicarbonate solution, and saturated sodium chloride solution with a concentration of 1mol / L, dried over anhydrous sodium sulfate, and then distilled under reduced pressure The solvent was removed to obtain a crude product, which was purified by flash column chromatography to obtain 8.6 g of the target intermedi...
Embodiment 2
[0028] The preparation of embodiment 2, L-proline amide derivative c
[0029]
[0030] A. Preparation of intermediate i: prepared according to the method described in Example 1, except that "compound f (4.9g, 20mmol) in anhydrous dichloromethane solution (15mL)" was replaced by "compound h (2.9g, 10mmol) Anhydrous dichloromethane solution (15mL)" to obtain 4.7g of the target intermediate i (white solid), with a yield of 95%. mp 209-210°C; [α] 20 D =-21.50 (c=0.1, CH 2 Cl 2 ); 1 HNMR (300MHz, CDCl 3 )(ppm)=1.63(s, 9H), 1.76(s, 2H), 1.87-2.07(m, 4H), 3.38(s, 3H), 3.69-3.73(m, 2H), 4.16(s, 2H) , 4.38-4.45(m, 1H), 5.12-5.35(d, 1H), 6.38-6.74(d, 1H), 7.38-7.43(m, 2H), 7.57-7.59(m, 2H), 7.75-7.78( d, 2H); 13 CNMR (75MHz, CDCl 3 ) (ppm) = 24.7, 25.7, 28.3, 30.7, 33.5, 46.7, 48.5, 80.6, 122.2, 123.4, 126.6, 127.2, 128.1, 131.1, 131.2, 131.3, 133.4, 133.8, 164.1, 165.5, HRMS (ESI (172.0; ): (C 28 h 33 o 5 N 3 Na) + The calculated value of is 514.2312 and the measured ...
Embodiment 3
[0032] The preparation of embodiment 3, L-proline amide derivative d
[0033]
[0034] A. Preparation of intermediate g: prepared according to the method described in Example 1.
[0035] B. Preparation of L-prolinamide derivative d: Intermediate g (440mg, 1mmol) and hydrazine hydrate (0.12mL) were refluxed in ethanol (5mL) for 2 hours; ) was diluted to precipitate phthalic hydrazide, filtered, and the filtrate was distilled off under reduced pressure to obtain a crude product. The crude product was dissolved with dilute hydrochloric acid (2mL), filtered, and the filtrate was adjusted to pH 7 with saturated sodium bicarbonate solution. , extracted 3 times with dichloromethane (each 20mL), combined the dichloromethane extracts, washed with saturated sodium bicarbonate solution, dried over anhydrous sodium sulfate, and then distilled off the solvent under reduced pressure to obtain the target product d (white solid ) 0.265g, yield 85%. 1 HNMR (300MHz, CDCl 3 )(ppm)=1.10-1.3...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com