Cough-relieving tablets and preparation method thereof
A technology of Keke Tablets and Plain Tablets, which is applied in the direction of medical formulas, medical preparations with no active ingredients, medical preparations containing active ingredients, etc., can solve the problems of unqualified tablet quality and poor effect, and achieve intermediate The effect of fewer links, low cost and low energy consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
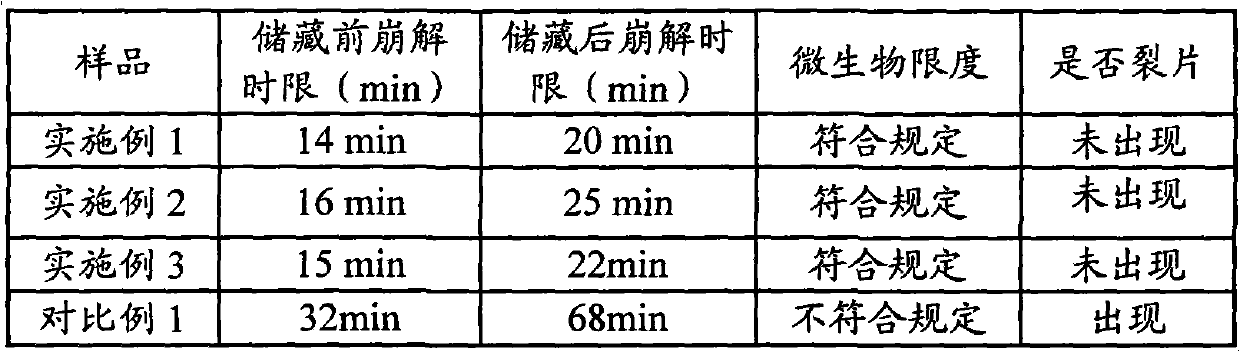
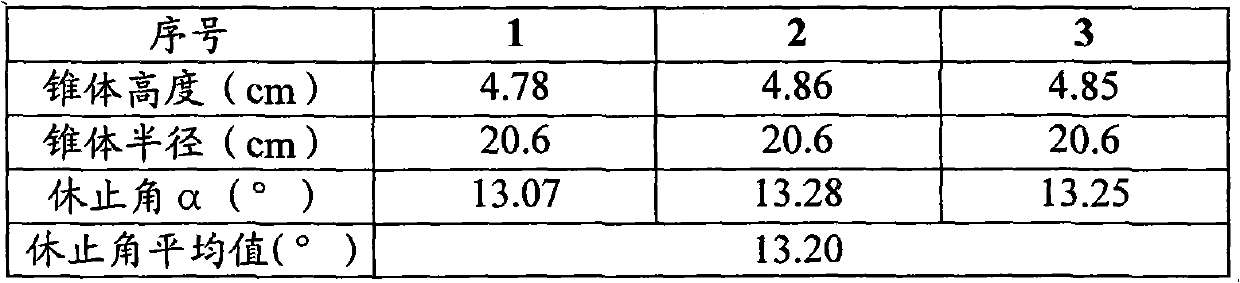
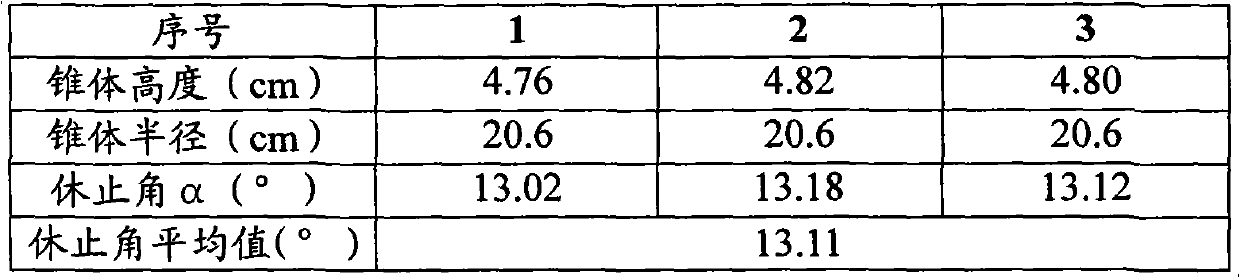
[0047] Take 240g of ephedra and 240g of poppy shell, crush them into powder respectively, pass through a 80-mesh sieve to obtain the fine powder and the remaining coarse powder, leave 110g of ephedra fine powder and 110g of poppy shell fine powder for later use; the remaining 130g of ephedra coarse powder and 130g of poppy shell Coarse powder is added with acidic aqueous solution (an acidic aqueous solution whose pH value is adjusted to 5 with 10% hydrochloric acid aqueous solution by volume percentage concentration) decocting twice, the water of 10 times of coarse powder weight is added for the first time, and 8 times of coarse powder is added for the second time. Powder weight water, decocted for 2 hours each time, filtered, and the filtrate was adjusted to pH 7 with 10% sodium hydroxide aqueous solution with a mass percentage concentration to obtain a medicinal solution for subsequent use; the remaining 240g licorice, 240g bitter almonds, and 75g radish Potatoes, 75g platyco...
Embodiment 2
[0051] The preparation of medicinal material extraction ointment powder is the same as that in Example 1.
[0052] Grind the lactose powder, pregelatinized starch, magnesium stearate, microcrystalline cellulose, silicon dioxide and polyethylene glycol 4000 through a 100-mesh sieve respectively, and set aside.
[0053] Put 290g of the above-mentioned lactose powder, 42.8g of pregelatinized starch and 193g of medicinal material extract paste powder into the mixer, start the machine and mix evenly to obtain a premix; add 43.8g of microcrystalline cellulose and 80g of polyethylene glycol 4000 to the In the above premix, mix well to obtain a mixture; put the above mixture into a GZL series dry roller compaction granulator, the pressure of the roller is 80Pa, the speed of the roller is 16 rpm, and the conveying speed of the mixture is 65 rpm. Start the machine, pass through a 40-mesh sieve after granulation, and discharge the material to obtain 613g of granular matter; add the above...
Embodiment 3
[0055] The preparation of medicinal material extraction ointment powder is the same as that in Example 1.
[0056] Grind the lactose powder, pregelatinized starch, magnesium stearate, microcrystalline cellulose, silicon dioxide and polyethylene glycol 4000 through a 100-mesh sieve respectively, and set aside.
[0057] Put 110.8g of the above-mentioned lactose powder, 39.6g of pregelatinized starch and 490g of medicinal material extract paste powder into the mixer, start the machine and mix evenly to obtain a premix; add 39.6g of microcrystalline cellulose and 60g of polyethylene glycol 4000 Put the above mixture into the above premix and mix it evenly to obtain the mixture; put the above mixture into the GZL series dry roller granulator, the pressure of the roller is 60Pa, the speed of the roller is 10 rpm, and the conveying speed of the mixture is 45 rpm , start the machine, pass through a 80-mesh sieve after granulation, and then get 630g of granular matter; add the above-me...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Mesh | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com