Preparation method and application of ZnO-doped TiO2 composite hollow sphere
A hollow sphere, carbon sphere technology, applied in chemical instruments and methods, metal/metal oxide/metal hydroxide catalysts, light water/sewage treatment, etc., to achieve separation, broaden the spectral response range, and reduce energy consumption. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0018] 1) Weigh 0.0022g of zinc acetate hexahydrate into a three-neck flask with stirring, measure 30ml of absolute ethanol, and prepare an ethanol solution of zinc acetate. Then weigh 0.06g of nano-carbon spheres, the diameter of which is in the range of 150-400nm, add 0.3ml of distilled water into it, and ultrasonically disperse until uniformly mixed.
[0019] 2) Add 30 ml of absolute ethanol into a dry constant pressure dropping funnel, measure 0.3 ml of n-butyl titanate and add it to prepare an ethanol solution of n-butyl titanate.
[0020] 3) Slowly add n-butyl titanate ethanol solution into the mixed solution obtained in step 1) under stirring condition, stir, and heat to reflux at 80° C. for 6 h. After the reflux is completed, continue to stir for 30 minutes, centrifuge, wash, and dry to obtain Zn 2+ Doped carbon / titania core-shell particles.
[0021] 4) with the Zn that step 3) obtains 2+ The doped carbon / titanium dioxide core-shell particles were fired in a mufur f...
example 2
[0025] 1) Weigh 0.0066g of zinc acetate hexahydrate into a three-neck flask with stirring, measure 50ml of absolute ethanol, and prepare an ethanol solution of zinc acetate. Then weigh 0.06g of nano-carbon spheres with a diameter ranging from 150 to 400nm, add 0.5ml of distilled water into it, and ultrasonically disperse until uniformly mixed.
[0026] 2) Add 50 ml of absolute ethanol into a dry constant-pressure dropping funnel, measure 0.3 ml of n-butyl titanate and add it to prepare an ethanol solution of n-butyl titanate.
[0027] 3) Slowly add n-butyl titanate ethanol solution into the mixed solution obtained in step 1) under stirring condition, stir, and heat to reflux at 80° C. for 6 h. After the reflux is completed, continue to stir for 6 hours, centrifuge, wash, and dry to obtain Zn 2+ Doped carbon / titania core-shell particles.
[0028] 4) with the Zn that step 3) obtains 2+ The doped carbon / titanium dioxide core-shell particles were burned in a mufur furnace at 55...
example 3
[0031] Operate according to the preparation process steps of specific example 2, the difference is that the amount of zinc acetate hexahydrate added in step 2) is 0.022g, and the TiO doped with ZnO is obtained 2 Hollow sphere, wherein the molar ratio of Zn / Ti in the hollow sphere is 1 / 10.
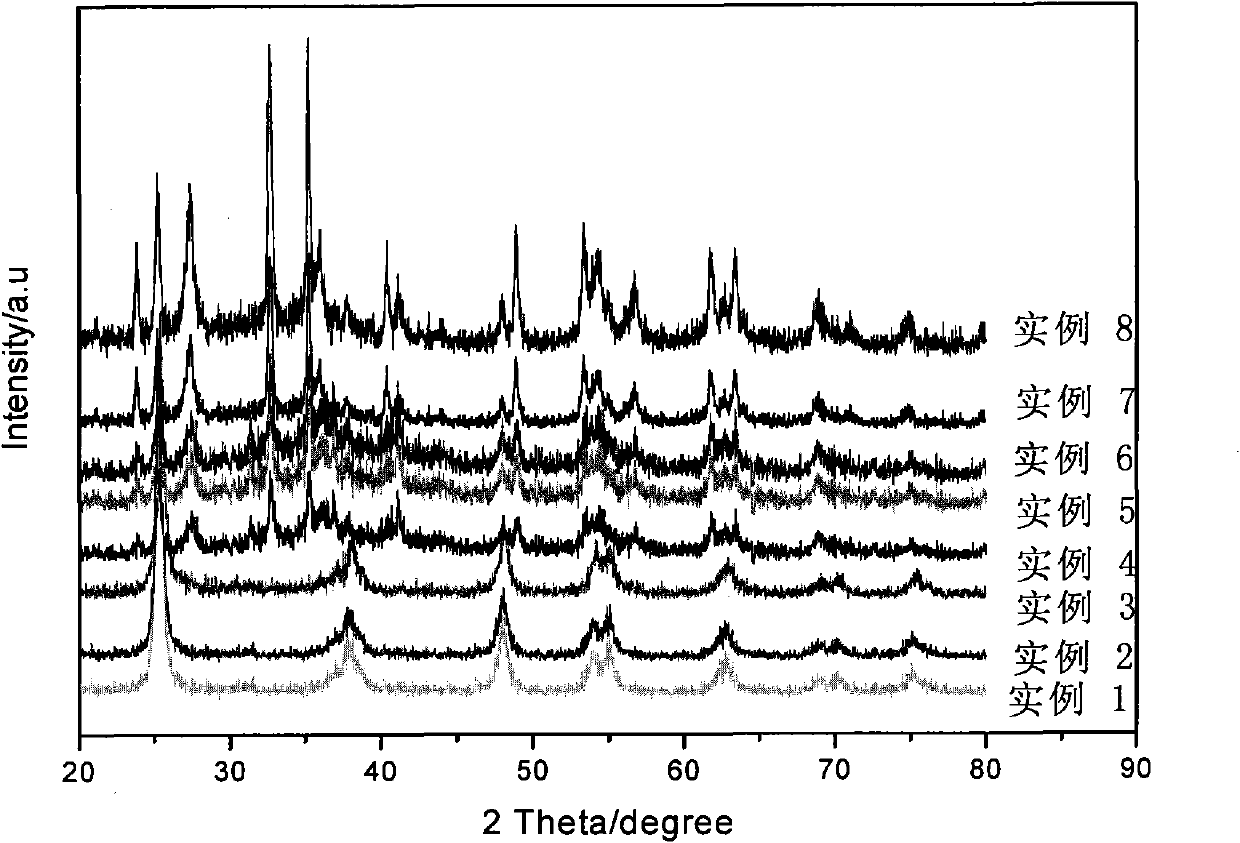
[0032] Attachment of the present invention figure 1 According to the curve of example 3, it is the XRD pattern of the photocatalyst that makes. It can be seen from the figure that the TiO in the composite photocatalyst 2 For the anatase structure.
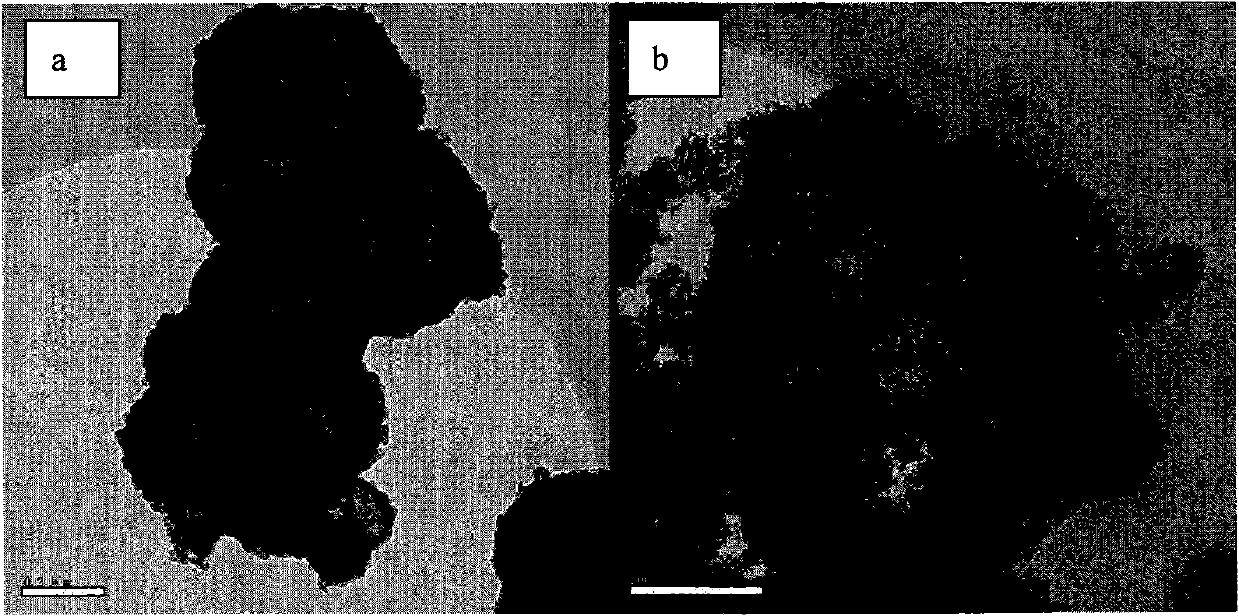
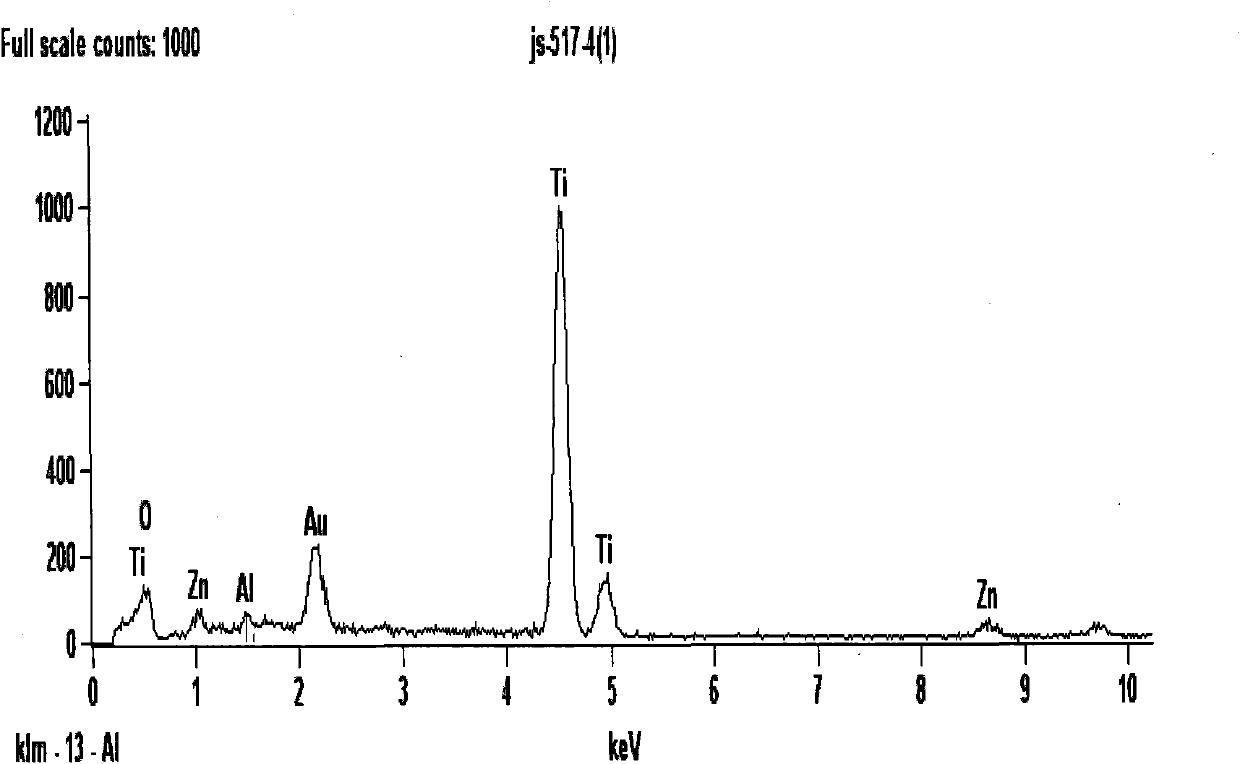
[0033] Attachment of the present invention image 3 It is the SEM picture of the photocatalyst prepared by Example 3. It can be seen from the figure that the prepared photocatalyst has a hollow spherical structure with an average diameter of 338.38nm, ranging from 200 to 481.18nm, and an average wall thickness of 39.3nm, ranging from 18.18 to 45.46nm. The specific surface area of the sample is 256.30m 2 / g, the photocatalytic degradation...
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com