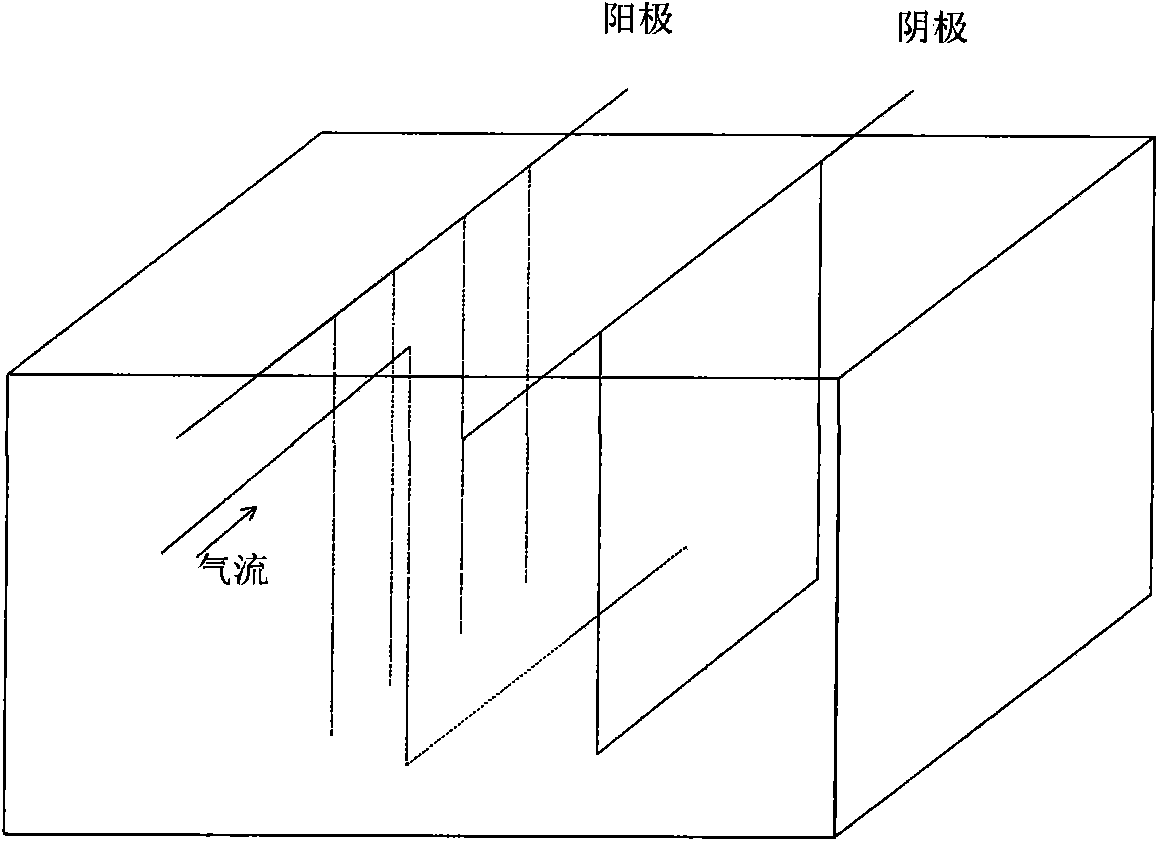
Method for recycling copper from copper nitrate waste water
A technology of copper nitrate and copper recovery, which is applied in the field of electrochemical applications, can solve the problems of large power consumption, different solution concentrations, and poor copper purity, and achieves the effects of simplicity and easy realization, simple production process, and improved recovery purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Embodiment 1: Fill about 400L of copper nitrate wastewater with a copper nitrate concentration of 53g / L into the electrolytic cell. The anode adopts 4 iron rods with a diameter of 16mm, and the negative electrode adopts a copper plate of 0.5 to 1mm. In the electrolytic cell, use a vortex The air pump continuously stirs the copper nitrate solution with a gas volume of 72m 3 / h, so that the copper nitrate wastewater is in a state of violent tumbling in the electrolytic cell, and the current density is 120A / m 2 Direct current, after about 25 hours of electrolysis, the copper nitrate concentration in the electrolytic mother liquor is 94mg / L (copper ion concentration is 32mg / L), 7.2kg of copper is recovered, the copper recovery rate is 99.83%, and the electric energy efficiency is 3.1KWh / Kg.
Embodiment 2
[0048] Embodiment 2: the copper nitrate concentration is that about 400L of copper nitrate waste water (that is, copper ion concentration is 26g / L) of 76g / L is charged in the electrolyzer, and the anode adopts 4 iron rods with a diameter of 16mm, and the negative electrode adopts 0.5~ 1mm copper plate, using the vortex air pump to continuously stir the copper nitrate solution in the electrolytic cell, the gas volume is 48m 3 / h, so that the copper nitrate wastewater is in a state of violent tumbling in the electrolytic cell, and the current density is 120A / m 2 DC current, after electrolysis for about 35 hours, the concentration of copper nitrate in the electrolytic mother liquor is 88mg / L (that is, the concentration of copper ions is about 30mg / L), 10.4kg of copper is recovered, the recovery rate of copper is 99.88%, and the power efficiency is 3.0KWh / Kg .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com