Method for synthesizing (2R, 3R)-3-phenyl ethylene oxide formic ether
A technology of phenyloxirane formate and synthesis method, which is applied in the field of side chain synthesis of anti-cancer raw materials docetaxel and paclitaxel, can solve problems such as unsuitability, and achieve high safety and simple raw materials Easy to obtain, no effects of highly toxic compounds
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0028] Preferred embodiments of the present invention are described below, and it should be understood that the preferred embodiments described here are only used to illustrate and explain the present invention, and are not intended to limit the present invention.
[0029]
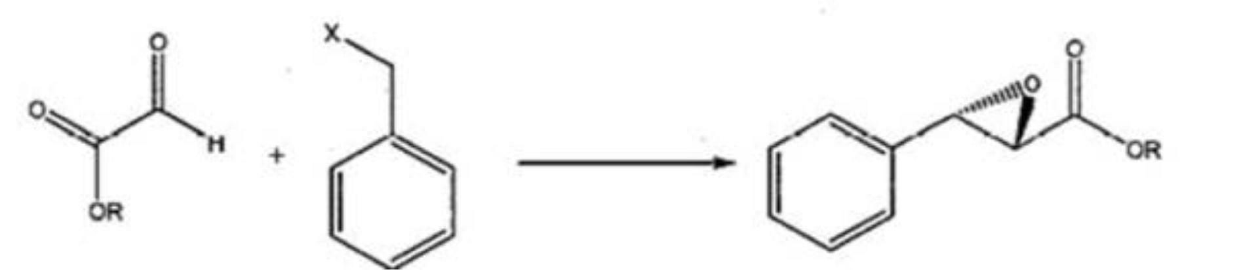
[0030] 1. Synthesis of (2R, 3R)-3-phenyloxirane ethyl carboxylate (compound 1):
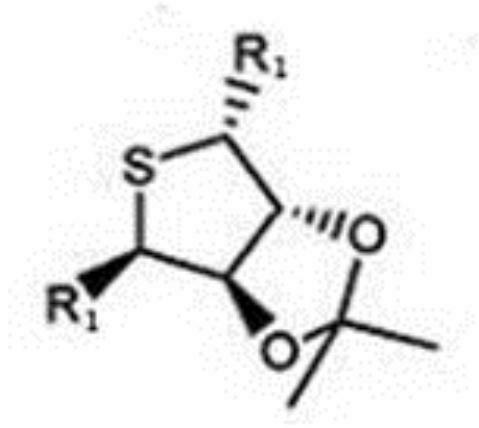
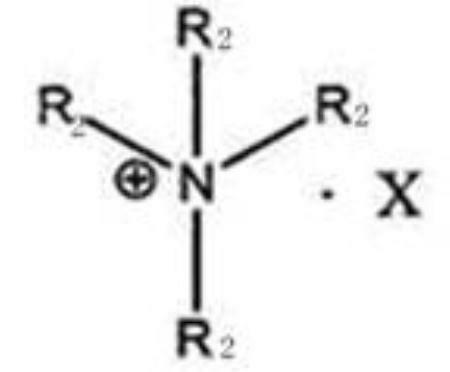
[0031] 204g (2mol) of ethyl glyoxylate, 424.25g (2.5mol) of benzyl bromide, 13.6g of tetrabutylammonium hydrogen sulfate, 160g of sodium hydroxide, 75.2g (0.43mol) of sulfolane derivatives in 15 in a mixed solvent of acetonitrile and water (9:1). After stirring at room temperature for 40 hours, filter, evaporate acetonitrile, add 4 liters of ethyl acetate, extract with 2 liters of water, and concentrate the organic phase to obtain an oily substance. After passing through a silica gel column, the mobile phase was ethyl acetate and petroleum ether (1:6), to obtain 296.4 g of compound 1, and the ee value determined by HPLC w...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com