2-(nitrogen heterocycle) benzimidazole complex alkyl aluminum compound, and preparation method and application thereof
A technology of benzimidazole and nitrogen heterocycle, applied in the field of organoaluminum compounds and its preparation, to achieve the effects of cheap and easy-to-obtain raw materials, high catalytic activity, and stable properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
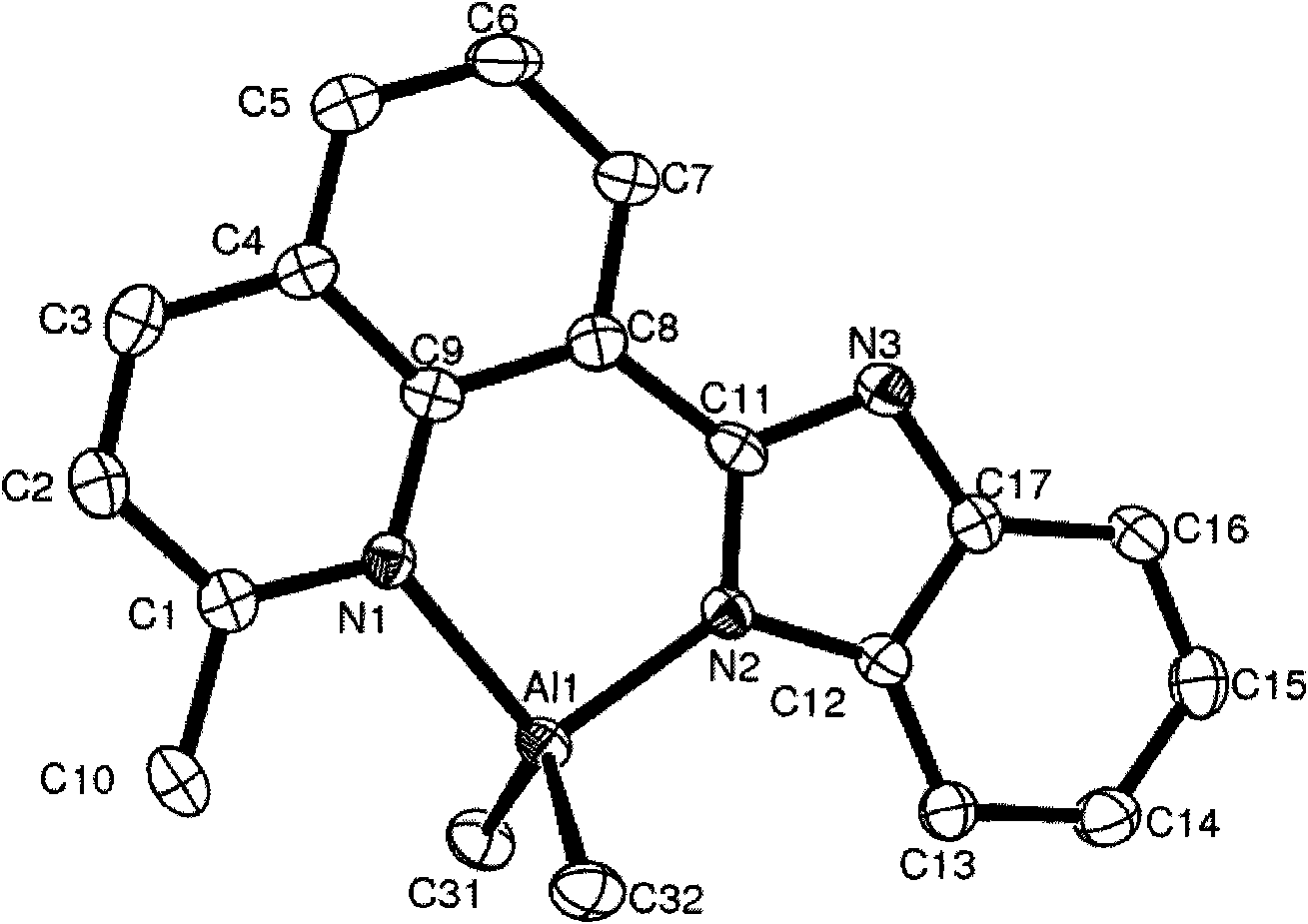
[0057] Embodiment 1, compound C1 (Me 2 Preparation of AlL1L1=2-methyl-8-benzimidazole-quinoline)
[0058] The ligand 2-methyl-8-benzimidazole quinoline (0.26g, 1.0mmol) and toluene (15mL) were added to a 100mL schlenk bottle, cooled to -30°C, and 1.0M trimethylaluminum was added with a syringe Toluene solution (1.0 mL, 1.0 mmol) was stirred, and the reaction was stirred at room temperature for 3 hours, and the volatile substances were removed under reduced pressure. Then add 5 mL of toluene to dissolve, add 15 mL of n-hexane, and recrystallize to obtain 0.28 g of compound C1 as a yellow powder, whose structural formula is shown in formula (a), yield: 88.4%.
[0059]
[0060] Characterization data of compound C1: 1 H NMR (CDCl 3 , ppm): δ9.64 (d, 1H, J = 7.35Hz, Quin-H), 8.49 (d, 1H, J = 8.40Hz, Quin-H), 7.94 (d, 1H, J = 7.67Hz, Quin -H), 7.82(m, 2H, Quin-H), 7.69(d, 1H, J=7.41Hz, Ar-H), 7.56(d, 1H, J=8.40Hz, Ar-H), 7.27(m , 2H, Ar-H), 3.23(s, 3H, Quin-CH 3 ), -0.39 (s...
Embodiment 2
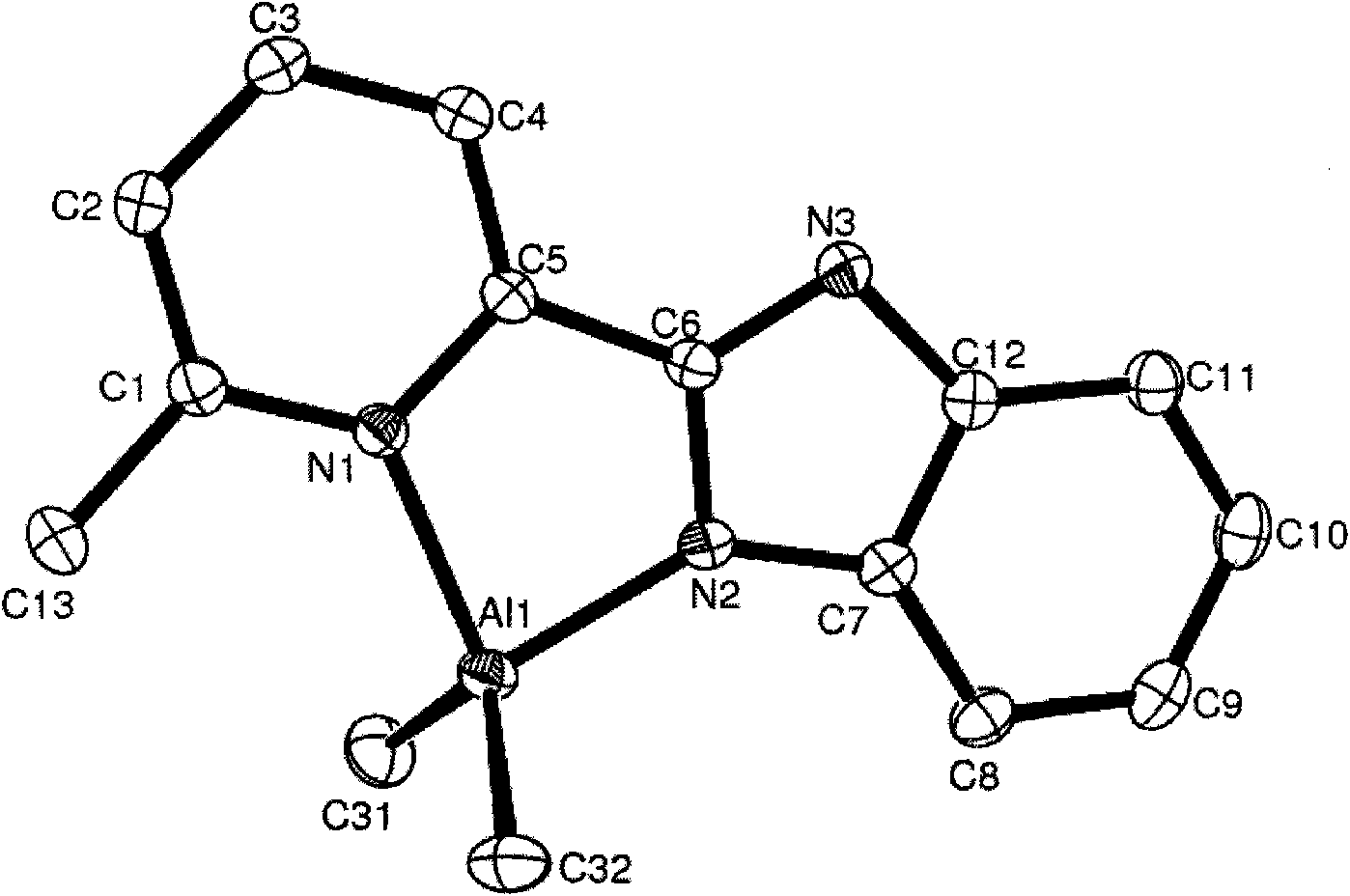
[0062] Embodiment 2, compound C2 (Et 2 Preparation of AlL1 L1=2-methyl-8-benzoimidazoquinoline)
[0063] The experimental procedure is the same as that in Example 1. The ligand 2-methyl-8-benzimidazolinoline was reacted with triethylaluminum in equimolar ratios to obtain compound C2, whose structural formula is shown in formula (b). Yield: 0.25 g (72.1%).
[0064]
[0065] Characterization data for compound C2: 1 H NMR (CDCl 3 , ppm): δ9.65 (d, 1H, J = 7.50Hz, Quin-H), 8.51 (d, 1H, J = 8.37Hz, Quin-H), 7.94 (d, 1H, J = 7.79Hz, Quin -H), 7.85(m, 2H, Quin-H), 7.70(d, 1H, J=7.32Hz, Ar-H), 7.58(d, 1H, J=8.39Hz, Ar-H), 7.27(m , 2H, Ar-H), 3.38(s, 3H, Quin-CH 3 ), 0.82(t, 6H, J=8.08Hz, -AlCH 2 C H 3 ), 0.30 (m, 4H, -AlC H 2 CH 3 ). 13 C NMR (CDCl 3C 21 h 22 AlN 3 : C, 73.45; H, 6.46; N, 12.24. Found: C, 73.73; H, 6.16; N, 12.18%.
Embodiment 3
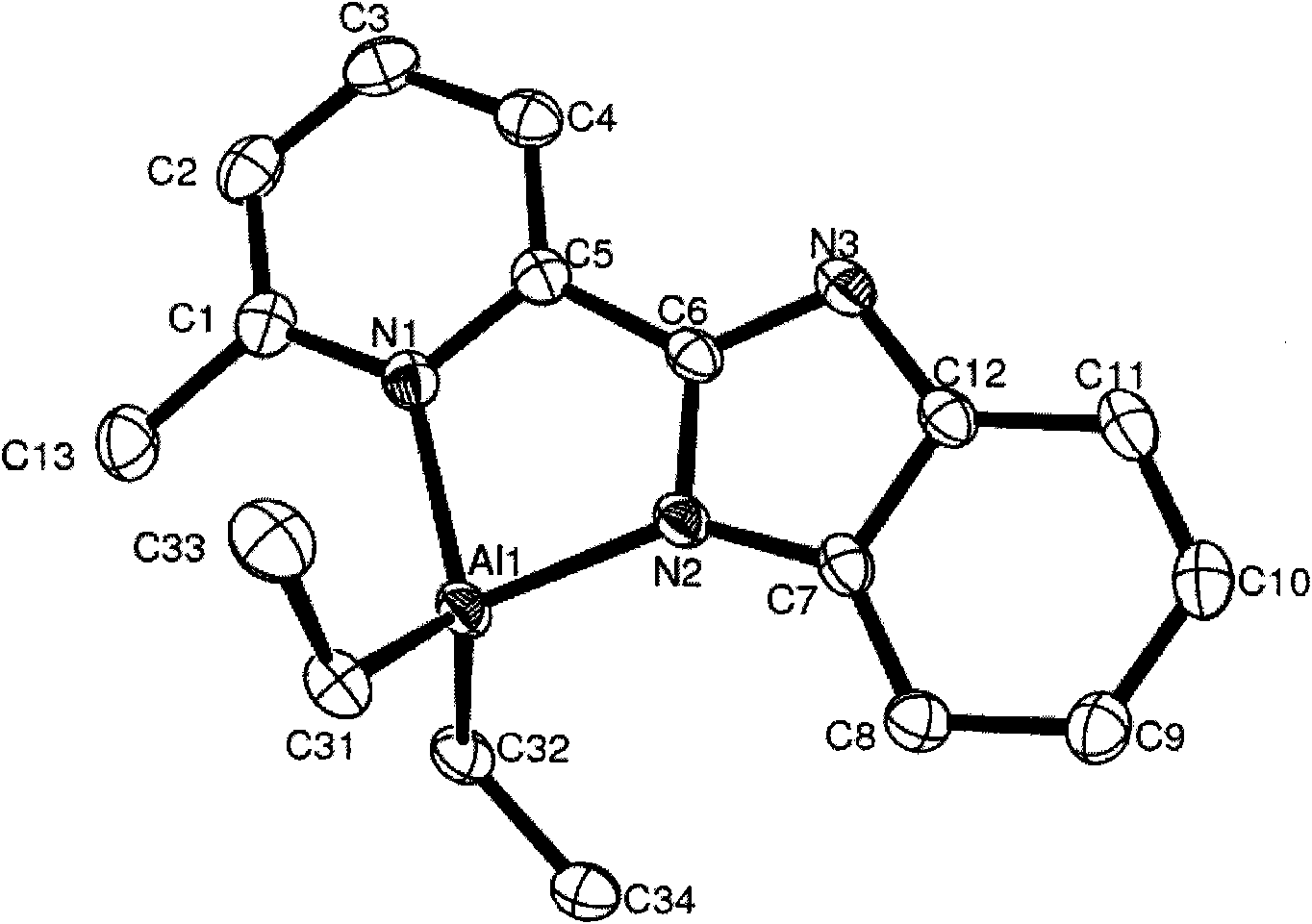
[0066] Embodiment 3, compound C3 ( i Bu 2 Preparation of AlL1 L1=2-methyl-8-benzoimidazoquinoline)
[0067] The experimental procedure is the same as that in Example 1. The ligand 2-methyl-8-benzimidazole quinoline reacts with triisobutylaluminum equimolarly to obtain compound C3, whose structural formula is shown in formula (c). Yield: 0.27 g (68.3%).
[0068]
[0069] Characterization data of compound C3: 1 H NMR (CDCl 3 , ppm): δ9.68 (d, 1H, J = 7.50Hz, Quin-H), 8.53 (d, 1H, J = 8.28Hz, Quin-H), 7.96 (d, 1H, J = 8.89Hz, Quin -H), 7.86(m, 2H, Quin-H), 7.75(d, 1H, J=6.81Hz, Ar-H), 7.59(d, 1H, J=8.54Hz, Ar-H), 7.23-7.30 (m, 2H, Ar-H), 3.30(s, 3H, Quin-CH 3 ), 1.58 (m, 2H, -CH 2 C H (CH 3 ) 2 ), 0.66 (d, 6H, J=6.35Hz, -CH 2 CH(CH 3 ) 2 ), 0.61 (d, 6H, J=6.35Hz, -CH 2 CH(CH 3 ) 2 ), 0.45(m, 2H, -C H 2 CH(CH 3 ) 2 ), 0.33(m, 2H, -C H 2 CH(CH 3 ) 2 ). 13 C NMR (CDCl 3 , ppm): δ162.3, 153.2, 145.9, 144.1, 142.1, 141.3, 134.9, 130.4, 128.6, 128.2, 126.6,...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Number average molecular weight | aaaaa | aaaaa |
| Number average molecular weight | aaaaa | aaaaa |
| Number average molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com