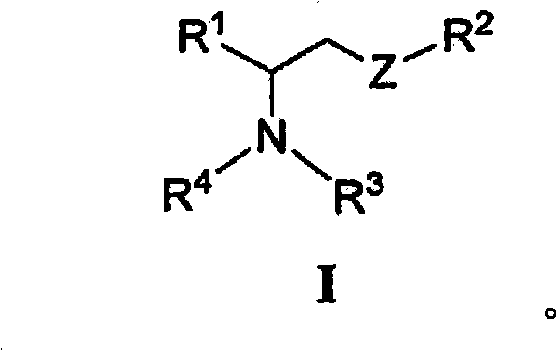
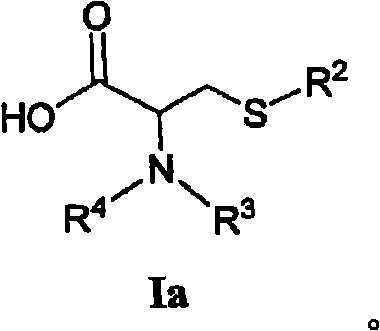
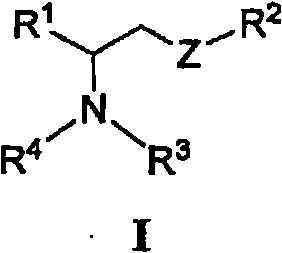
Acetyl mimic compounds for the inhibition of isoprenyl-s-cysteinyl methyltransferase
A compound and alkenyl technology, applied in drug combination, organic chemistry, digestive system, etc., can solve problems such as edema and fluid retention
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 20
[0282]
[0283] or a pharmaceutically acceptable salt, enantiomer, diastereomer or double bond isomer thereof, wherein:
[0284] R 1 is optionally substituted heteroaryl or:
[0285]
[0286]
[0287] R 2 is via one or more R 7 A radical substituted aliphatic group;
[0288] R 13 independently for H, or -NH-S(O) 2 R 14 ;
[0289] R 14 are independently H, (C1-C4) alkyl or aryl;
[0290] R 5 are independently H, alkyl, aryl, alkenyl or alkynyl, wherein R 5 Optionally one or two R 7 group substitution;
[0291] R 6 is H, alkyl, aryl, alkenyl, alkynyl or cyclic group, where R 6 Optionally one or two R 7 group substitution;
[0292] R 7 is-NHC(=O)(C 1 -C 8 ) Alkyl, -(C 1 -C 8 ) Alkyl, -(C 1 -C 8 ) alkenyl, -(C 1 -C 8 ) alkynyl, phenyl, -(C 2 -C 5 ) heteroaryl, -(C 1 -C 6 ) heterocycloalkyl, -(C 3 -C 7 ) cycloalkyl, -O-(C 1 -C 8 ) Alkyl, -O-(C 1 -C 8 ) alkenyl, -O-(C 1 -C 8 )alkynyl, -O-phenyl, -CN, -OH, oxo, halo, -C(=O)OH, -COhalo, ...
example
[0339] As described in the Examples below, in certain exemplary embodiments, compounds are prepared according to the following general procedures. It is to be understood that while the general methods describe the synthesis of certain compounds of the invention, the following general methods, and others known to those skilled in the art, are applicable to all classes, subclasses and classes of each of the described compounds disclosed herein. type.
[0340] The following general experimental procedure was used in each of the examples described below. Proton NMR ( 1 HNMR) spectrum is recorded on the Bruker 500MHz spectrometer, dimethyl sulfoxide (DMSO-d6), methanol (CD 3 0D) or chloroform (CDCl 3 ) used as 1 H-NMR solvent. The residual proton absorption of the deuterated solvent was used as an internal standard. all 1 H-NMR chemical shifts are reported as delta values in parts per million (ppm). Splitting patterns are abbreviated as follows: s, singlet; d, doublet; t,...
example 1
[0342] Synthetic compounds (where R 3 = pyridine derivatives)
[0343]
[0344] 2-Bromopyridine was added to a solution of farnesylcysteine methyl ester in diglyme and heated to 130°C under nitrogen for 19 hours. The reaction mixture was diluted with aqueous ammonia, and the product was extracted into chloroform. The combined organic phases were dried over anhydrous sodium sulfate and the solvent was removed in vacuo to give the crude product which could be purified using reverse phase HPLC. The protective methyl group on -COOH can be hydrolyzed under basic conditions before or after purification. Details of this method are described in Ito et al., Biological & Pharmaceutical Bulletin. 2007 30: pp. 1838-1843.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com