Method for preparing nano superfine rare-earth hexaboride powder
The technology of hexaboride and ultrafine powder is applied in the field of preparation of rare earth hexaboride nanometer ultrafine materials, which can solve the problems of high cost of reaction vessel, difficulty of hexaboride and high product cost, and achieves low pressure and low price. , the effect of good appearance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Embodiment 1. synthetic LaB 6
[0045] The source of lanthanum is lanthanum chloride and lanthanum oxychloride, with LaCl 3 Based on the LaOCl mixture, the molar ratio is 5:1. After uniformly mixing 156g of lanthanum source, 250g of magnesium powder, and 223g of boron oxide, they are placed in a 500mL reactor. The opening was compacted, placed in a resistance crucible boiler, and reacted at 600 ° C for 10 hours; the reactor was naturally cooled to room temperature; the initial product and ice were mixed according to a mass ratio of 1:2, and then a small amount of hydrochloric acid (hydrochloric acid 0.3 times Mg molar amount in the raw material), after stirring for 10 minutes, add hydrochloric acid in several times to make the final concentration of hydrochloric acid in the system 3mol / L, and the temperature is ≤95°C; heat the solution containing the initial product after preliminary hydrochloric acid treatment and maintain it at 50-90 °C, after 1-5 hours of treatment...
Embodiment 2
[0047] Example 2. Synthesis of CeB 6
[0048] The source of cerium is cerium chloride and cerium oxide, with CeO 2 and CeCl 3 According to the molar ratio of 1:6, 0.80g of cerium source, 1.25g of magnesium powder, and 1.02g of boron oxide were uniformly mixed, put in a 25mL reactor, and kept at 600°C for 10 hours, because the amount of the initial product was relatively small , The heat generated by the use of hydrochloric acid and the initial product is relatively small compared to the system, which will not cause the system to boil, so it is only acid-washed with 3 mol of hot hydrochloric acid (40 ° C), filtered, washed with alcohol solvent, and vacuum-dried. Obtain cubic CeB with an average particle size of 50 nm or less 6 . Figure 4 For its XRD, due to the small particle size of the obtained product, the diffraction peaks are slightly shifted to high angles, but ten of the peaks can be indexed as CeB in the body-centered cubic system 6 According to Scherrer's formula...
Embodiment 3
[0049] Embodiment 3. synthetic LaB 6
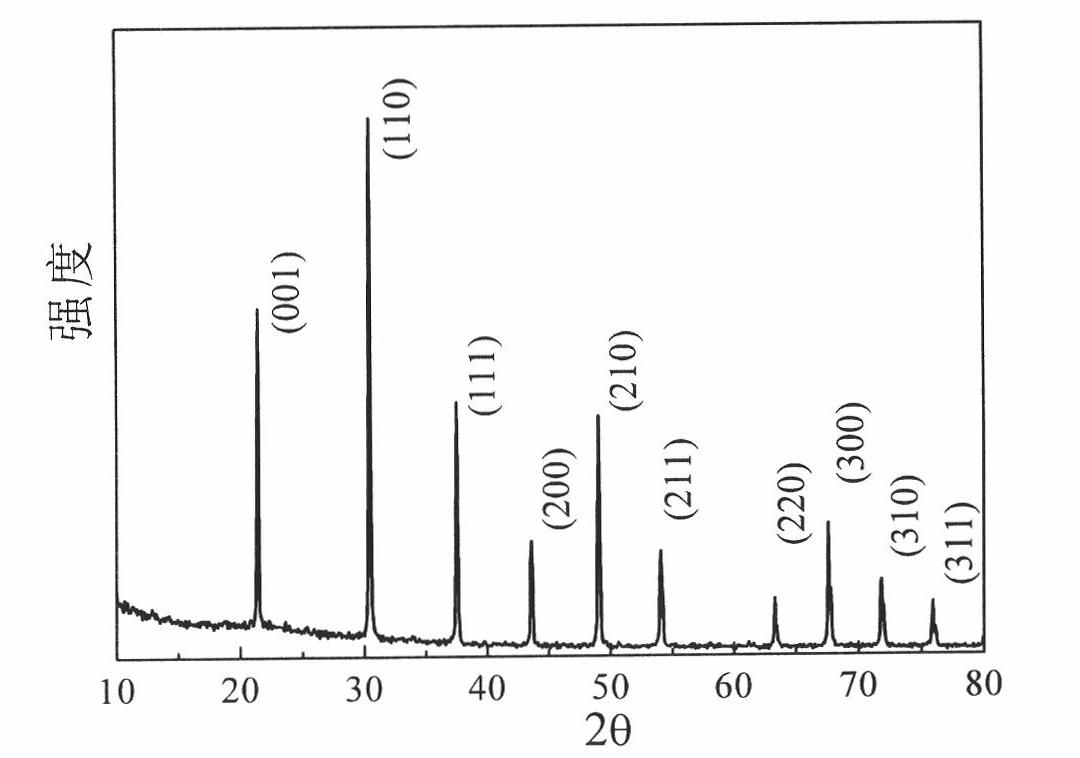
[0050] Lanthanum source is lanthanum chloride, LaCl 3 2.474g, 2.076g of magnesium powder, and 1.715g of boron oxide were mixed, placed in a 20mL reactor, and kept at 650°C for 8 hours. Since the amount of the initial product is relatively small, the heat generated by using hydrochloric acid and the initial product is relatively small, thus the system temperature will not be caused to rise sharply. Therefore, after it is cooled down to room temperature, it is treated in hot dilute hydrochloric acid (45 ° C), filtered after pickling, washed with alcohol, and dried in vacuum to obtain a compound containing LaB 6 of powder. Image 6 It is the X-ray diffraction spectrum of the product obtained in Example 3. Depend on Image 6 It can be seen that the product contains LaB 6 The product, the weaker impurity peak is LaOCl.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com