Method for preparing medicament carrying controlled-release nanometer material and application
A nano-material and drug-carrying technology, which is applied in pharmaceutical formulations, medical preparations of non-active ingredients, powder delivery, etc., can solve problems such as complex operation and silane coupling agent safety to be studied, and achieve strong biocompatibility , Improve drug bioavailability, effect of small particle size
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] step 1)
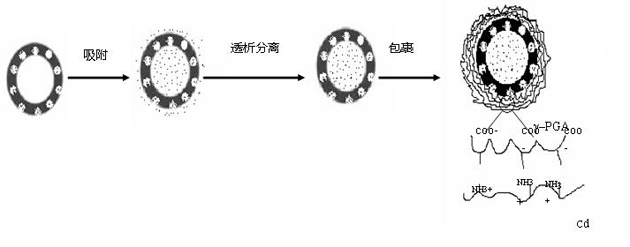
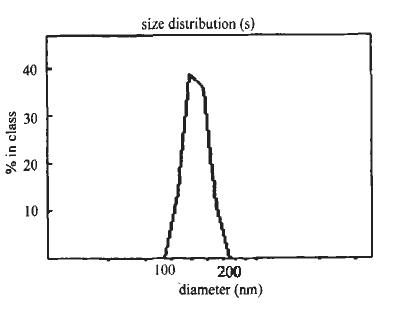
[0033] Prepare an aqueous solution of doxorubicin at a concentration of 5 mg / mL. Suction 3mL of silica nano-solution (average particle size 100nm, average shell thickness 15nm, shell average pore size 10nm, concentration 10mg / mL) for ultrasonic dispersion. The aqueous solution of doxorubicin was dripped (1ml / min) into the silica nano-solution, and after stirring at room temperature for 24 hours, the free drug was removed by dialysis to obtain the drug-loaded sandwich silica nano-solution.
[0034] step 2)
[0035] To prepare a polymer solution, first dissolve γ-PGA in deionized aqueous solution at a concentration of 1 mg / mL. At the same time, chitosan was dissolved in 1% acetic acid solution with a concentration of 1 mg / mL.
[0036] Inject the γ-PGA solution dropwise into step 1) to make the concentration of γ-PGA in the system reach 0.1% mg / mL, stir at room temperature for 1 hour, centrifuge the product, and wash it with deionized water for 2-3 times to fu...
Embodiment 2
[0040] Use a sandwich silica nanometer with an average particle size of 75nm (the average shell thickness is 12nm, the average pore diameter of the shell layer is 5nm, and the concentration is 9mg / mL) to replace the sandwich silica nanometer in step 1, and the concentration of doxorubicin is 1mg / mL instead of the doxorubicin concentration in step 1. Repeat the adsorption and washing process in step 2 alternately until four bilayer outer layers of γ-polyglutamic acid and chitosan membranes are obtained.
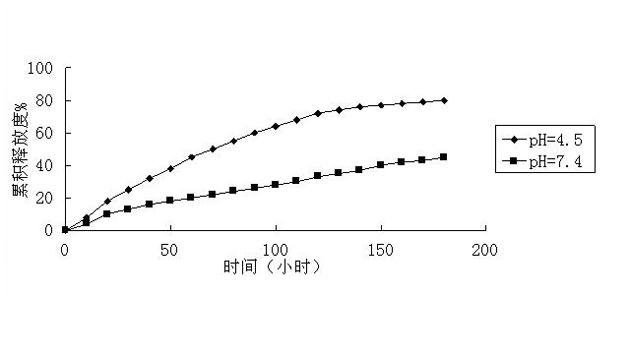
[0041] The sustained-release performance evaluation of the drug was the same as that in Example 1. The results showed that when pH=7.4, 25% was released within 180 hours; when pH=5, 30% was released within 180 hours. The composite support material is thus pH responsive. The drug loading of the composite carrier is 40%.
Embodiment 3
[0043] Use sandwich silica nanometers with an average particle size of 260nm (the average shell thickness is 20nm, the average pore diameter of the shell layer is 15nm, and the concentration is 15mg / mL) to replace the sandwich silica nanometers in step 1, and the concentration of doxorubicin is 15mg / mL instead of the doxorubicin concentration in step 1. The adsorption and washing process in step 2 were alternately repeated until three bilayer outer layers of γ-polyglutamic acid and chitosan films were obtained.
[0044] The sustained-release property evaluation of the drug was the same as in Example 1, and the results showed that, when pH=7.4, 65% was released within 180 hours; when pH=5, 50% was released within 180 hours. The composite support material is thus pH responsive. The drug loading of the composite carrier is 60%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
| Shell thickness | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com