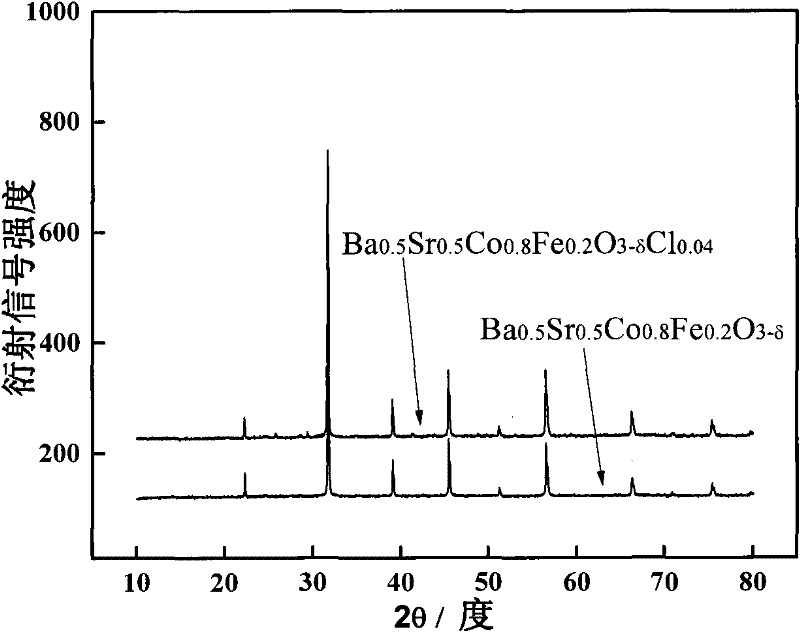
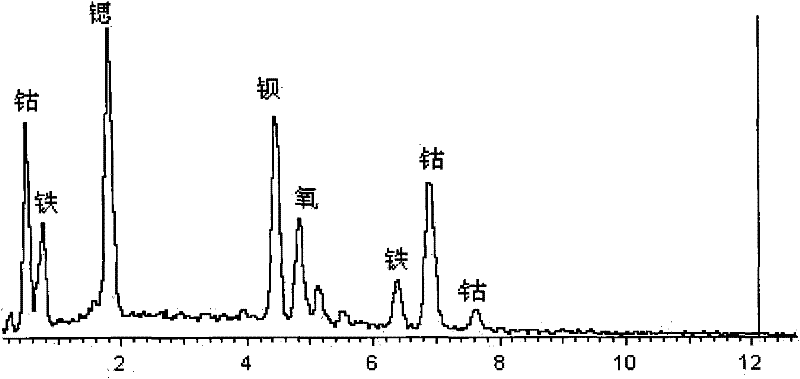
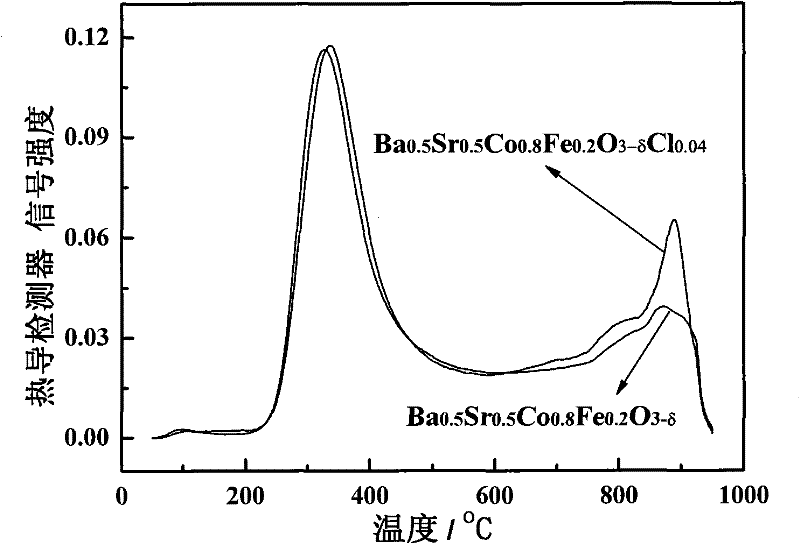
Halogen doped perovskite composite oxide catalyst as well as preparation method and application thereof
A composite oxide and perovskite-type technology, applied in the direction of physical/chemical process catalysts, condensation hydrocarbons with dehydrocarbons, chemical instruments and methods, etc., can solve the problem of low activity, achieve enhanced catalytic activity, Avoid the effect of loss and deep oxidation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] 0.1 mol of Ba was prepared by EDTA-citric acid method 0.5 Sr 0.5 co 0.8 Fe 0.2 o 3-δ Cl 0.04 (abbreviated as BSCFCl 0.04 , where δ=0~0.8) powder: take 0.5mol / l Ba(NO 3 ) 2 Solution 100ml, 0.5mol / l Sr(NO 3 ) 2 Solution 100ml, 0.5mol / l Co(NO 3 ) 2 Solution 160ml, 0.2mol / l Fe(NO 3 ) 3 Solution 90ml, 0.1mol / l FeCl 2 Solution 20ml is in the beaker of 2000ml, and according to total metal ion mole number: EDTA (ethylenediaminetetraacetic acid) mole number: the ratio of citric acid mole number=1:1:2 takes by weighing EDTA 58.45g, citric acid 84.06g adds Add deionized water to the beaker to 1500ml, then add concentrated ammonia water to adjust the pH to 6, stir with a glass rod until the aqueous solution becomes clear, and finally place it on the magnetic stirring heater at a rotor speed of 500r / min at a constant temperature of 80°C Water was removed by evaporation until a dark red gel formed. The prepared gel was transferred to a 1000ml evaporating dish, placed o...
Embodiment 2
[0036] 0.1 mol of Ba was prepared by EDTA-citric acid method 0.5 Sr 0.5 co 0.8 Fe 0.2 o 3-δ Cl 0.08 (abbreviated as BSCFCl 0.08 , where δ=0~0.8) powder: take 0.5mol / l Ba(NO 3 ) 2 Solution 100ml, 0.5mol / l Sr(NO 3 ) 2 Solution 100ml, 0.5mol / l Co(NO 3 ) 2 Solution 160ml, 0.2mol / l Fe(NO 3 ) 3 Solution 80ml, 0.1mol / l FeCl 2Put 40ml of the solution in a 2000ml beaker, and weigh 58.45g of EDTA and 84.06g of citric acid into the beaker according to the ratio of total moles of metal ions: moles of EDTA: moles of citric acid=1:1:2, and then add deionized water to 1500ml, then add concentrated ammonia water to adjust the pH to 6, stir with a glass rod until the aqueous solution becomes clear, and finally place it on a magnetic stirring heater at a rotor speed of 500r / min, evaporate the water at a constant temperature of 80°C until a deep red color is formed gel. The prepared gel was transferred to a 1000ml evaporating dish, placed on an electric furnace in a fume hood and ...
Embodiment 3
[0038] 0.1 mol of Ba was prepared by EDTA-citric acid method 0.5 Sr 0.5 co 0.8 Fe 0.2 o 3-δ Cl 0.12 (abbreviated as BSCFCl 0.12 , where δ=0~0.8) powder: take 0.5mol / l Ba(NO 3 ) 2 Solution 100ml, 0.5mol / l Sr(NO 3 ) 2 Solution 100ml, 0.5mol / L Co(NO 3 ) 2 Solution 160ml, 0.2mol / l Fe(NO 3 ) 3 Solution 70ml, 0.1mol / l FeCl 2 Solution 60ml is mixed in the beaker of 2000ml to obtain mixed solution, and take by weighing EDTA58.45g, citric acid 84.06g in the beaker according to the ratio of metal ion total moles: EDTA moles: citric acid moles=1:1:2, Then add deionized water to 1500ml, then add concentrated ammonia water to adjust the pH to 6, stir with a glass rod until the aqueous solution becomes clear, and finally place it on the magnetic stirring heater at a rotor speed of 500r / min, and evaporate at a constant temperature of 80°C to remove Moisture until a dark red gel forms. The prepared gel was transferred to a 1000ml evaporating dish, placed on an electric furnace ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com