Method for preparing lithium gallate crystals
A technology of lithium gallate and crystal, applied in the field of preparation of lithium gallate crystal, can solve the problems of serious melt volatilization, deviation of Li and Ga ratio from stoichiometric ratio, and high cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
[0022] An embodiment of preparing lithium gallate crystals provided by the present invention includes:
[0023] Will Li 2 CO 3 and Ga 2 o 3 As raw materials mixed and compacted to obtain a molded body, the molded body is sintered;
[0024] Heating the molded body under vacuum conditions to obtain a melt, dipping the seed crystal into the cold core of the melt while rotating the seed crystal, shouldering and pulling at equal diameters, and cooling the melt to grow lithium gallate at the same time crystals.
[0025] According to the present invention, select Li 2 CO 3 and Ga 2 o 3 Powder is used as raw material, among which, Li 2 CO 3 The purity is preferably greater than 99.99wt%, and the powder particle size is preferably less than 120 μm, more preferably less than 180 μm, more preferably less than 50 μm; Ga 2 o 3 The purity is preferably greater than 99.99 wt%, and the particle size of the powder is preferably less than 120 μm, more preferably less than 180 μm, mo...
Embodiment 1
[0035] According to molar ratio Li 2 CO 3 : Ga 2 o 3 =1.05:1 Weigh Li with a purity of 99.999% 2 CO 3 Powder and Ga 2 o 3 Powder, the particle size of the two powders is 50 μm to 70 μm. After the two powders were mixed in a mixer for 48 hours, they were pressed into a molded body with a diameter of 80 mm, and the pressing pressure was 1 GPa. The molded body was placed in a crucible, and sintered in a muffle furnace at a sintering temperature of 1200° C., a sintering time of 15 hours, and a sintering atmosphere of nitrogen. After sintering, the molded body is cooled to room temperature in the muffle furnace and taken out, then put into a molybdenum crucible with a diameter of 92mm, and then the molybdenum crucible is placed in the single crystal furnace, and the single crystal furnace is evacuated to 1×10 -3 Pa.
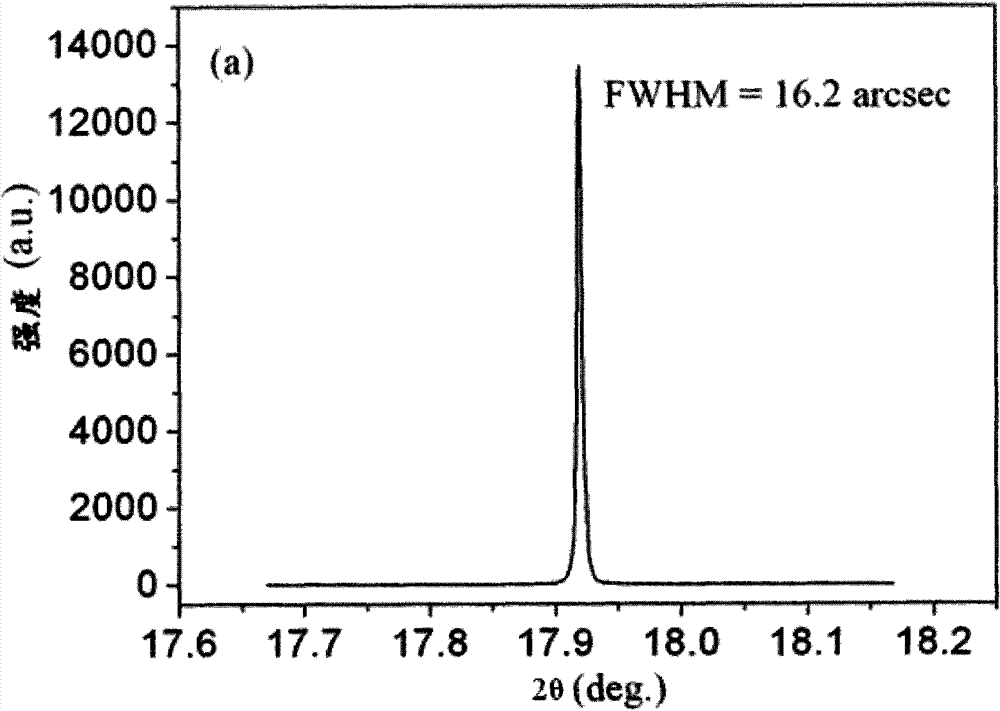
[0036] Raise the temperature of the single crystal furnace to 1750°C to melt the molded body to obtain a melt. After 40 minutes of heating, the vacuum in the...
Embodiment 2
[0041] The equipment used in this example is the same as in Example 1.
[0042] Take the molded body after sintering in Example 1, put it in a molybdenum crucible, then place the molybdenum crucible in the single crystal furnace, and evacuate the single crystal furnace to 1×10 -3 Pa.
[0043] Raise the temperature of the single crystal furnace to 1750°C to melt the molded body to obtain a melt, and take the seed crystal of the [001] crystal orientation. Slowly lower the seed crystal into contact with the cold core of the melt, and at the same time rotate the seed crystal at a speed of 15r / min, soak the seed crystal in the cold core for 30 minutes and start to shoulder. When putting the shoulders, the lifting speed is 1mm / h, and the power drop speed is 4.5w / h.
[0044] After shouldering, lithium gallate crystals are grown by equal diameter pulling, the seed crystal pulling speed is 5mm / h, and the power drop speed is 5w / h.
[0045] After the growth of the equal-diameter part ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com