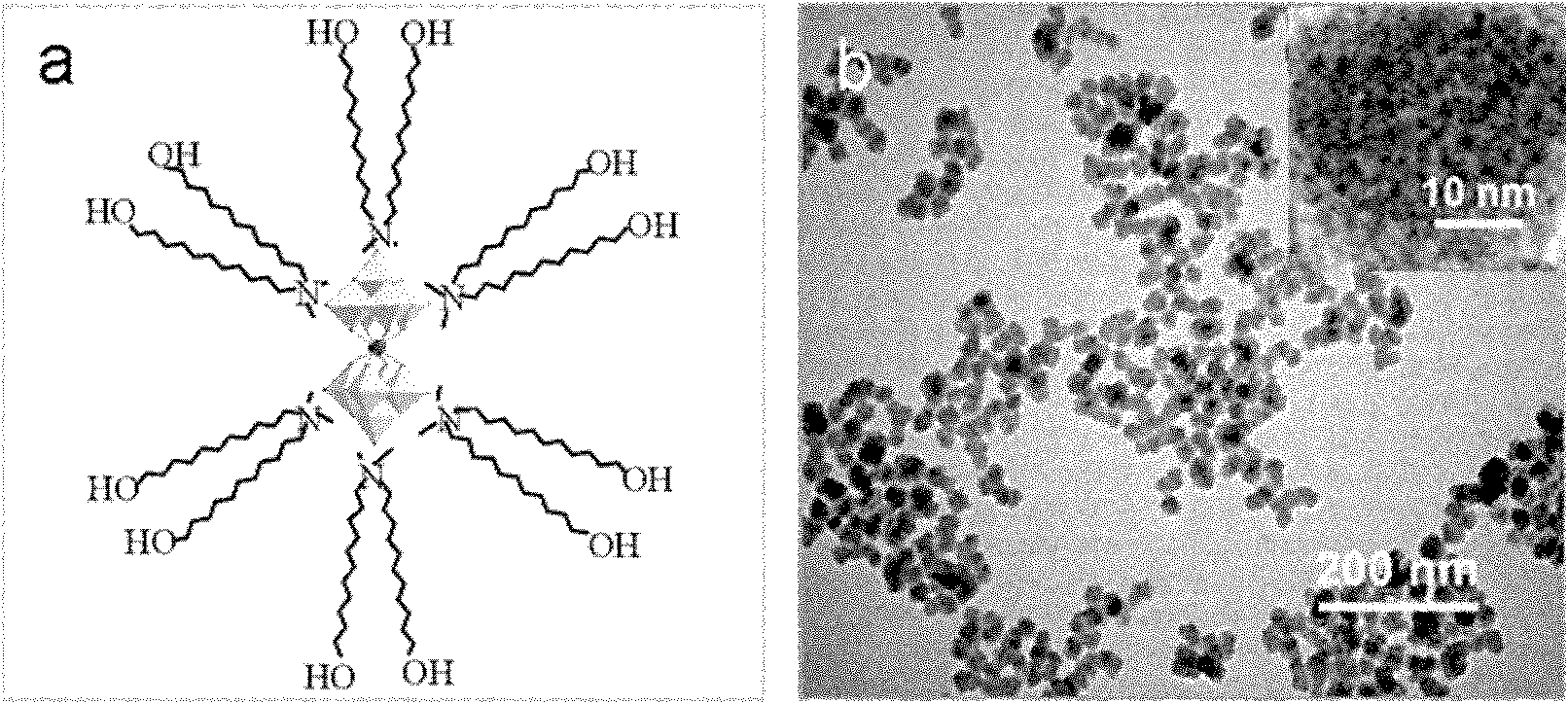
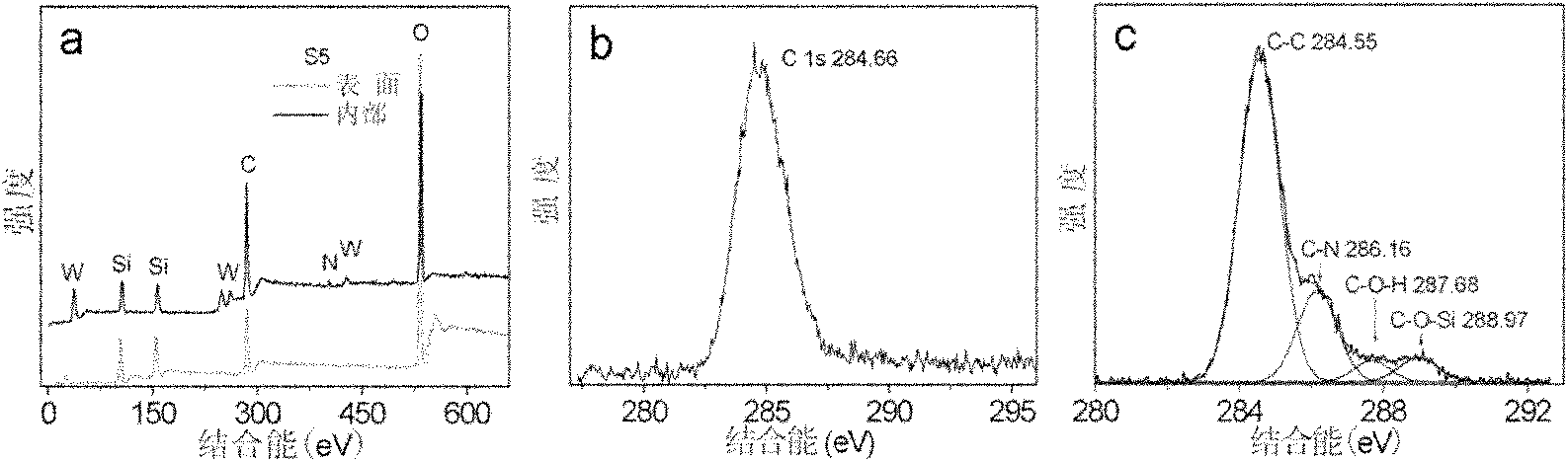
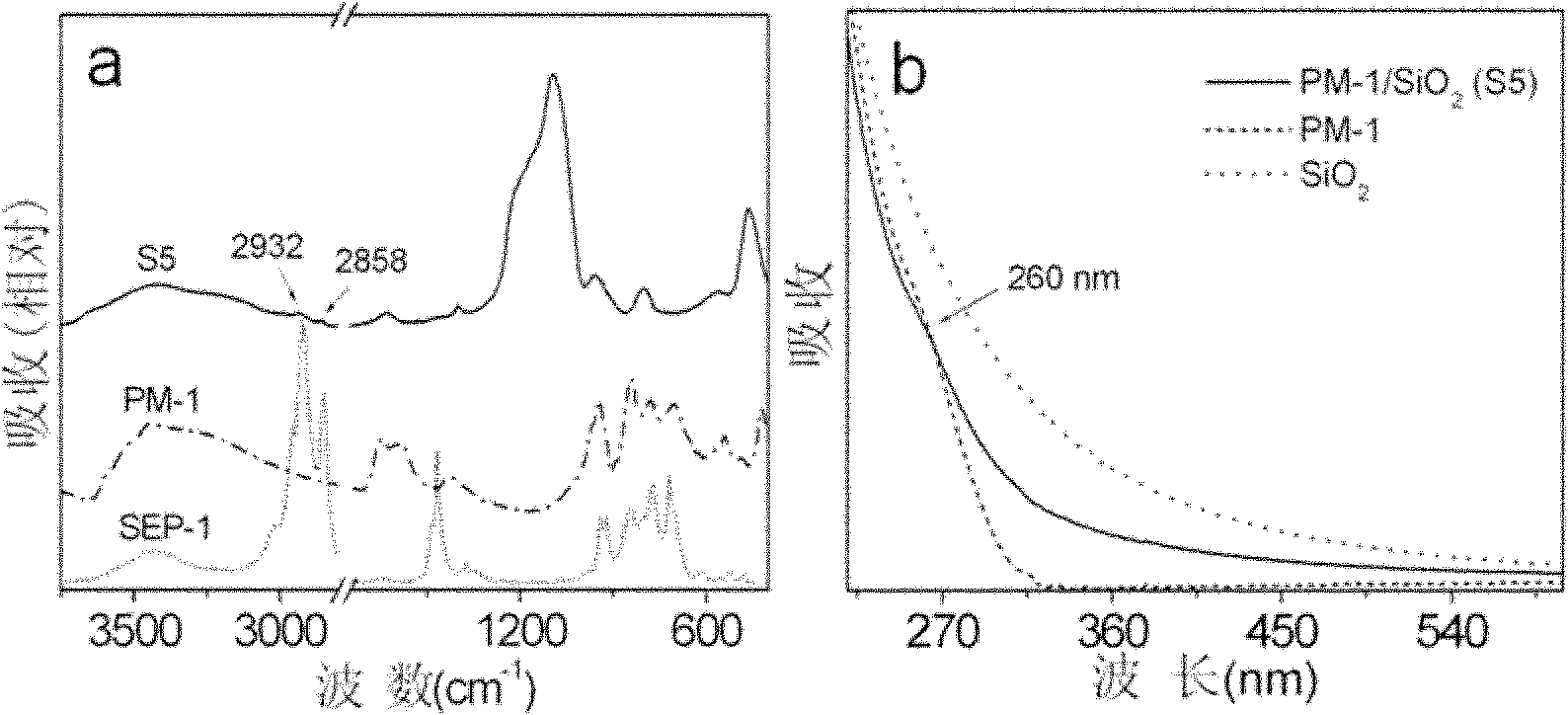
Method for preparing multi-metal-oxygen cluster-doped silicon dioxide nanoparticles
A technology of polymetallic oxygen clusters and nanoparticles, applied in the direction of nanotechnology, nanotechnology, nanostructure manufacturing, etc., can solve the problems of instability, limited universality, easy decomposition, etc., to achieve size controllable, leakage suppression, Good effect of monodispersity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-1
[0039]Example 1-1: N, N-dimethylbis-(11-hydroxyl-undecyl) ammonium bromide (DOHDA for short) coated Na 9 [EuW 10 o 36 ](PM-1)
[0040] Dissolve 0.2 g DOHDA in 20 mL deionized water, 0.14 g Na 9 wxya 10 o 36 Dissolve in 20 ml deionized water, DOHDA and Na 9 wxya 10 o 36 The molar ratio is 9:1 (the charge ratio is 9:9). Under stirring, will contain Na 9 wxya 10 o 36 The aqueous solution of DOHDA was added dropwise to the aqueous solution of DOHDA. After the dropwise addition, continue to stir for 5 hours, filter the resulting precipitate with a sand funnel, and dry the white solid in vacuum at room temperature to obtain DOHDA-embedded Na 9 [EuW 10 o 36 ] Complex (abbreviated as SEP-1), the yield was 60%. SEP-1 elemental analysis: C% 35.45, H% 6.81, N% 1.52, the chemical formula of SEP-1 is (DOHDA) 6 wxya 10 o 36 4H 2 O.
Embodiment 1-2
[0041] Example 1-2: 11-Hydroxy-Undecyl-Dimethyl Ammonium Hydrogen Bromide (referred to as HUDAH) coating K 12 [EuP 5 W 30 o 110 ](PM-2)
[0042] Dissolve 0.2 g of HUDAH in 20 mL of deionized water, 0.24 g of K 12 [EuP 5 W 30 o 110 ] dissolved in 20 ml deionized water, HUDAH and K 12 [EuP 5 W 30 o 110 ] The molar ratio is 12:1 (the charge ratio is 12:12). Under stirring, will contain K 12 [EuP 5 W 30 o 110 ] was added dropwise to the aqueous solution of HUDAH. After the dropwise addition, continue to stir for 5 hours, filter the resulting precipitate with a sand funnel, obtain a white solid and dry it in vacuum at room temperature to obtain HUDAH-embedded K 12 [EuP 5 W 30 o 110 ] Complex (abbreviated as SEP-2), the yield was 55%. SEP-2 elemental analysis: C% 18.00, H% 3.26, N% 1.60, the chemical formula of SEP-2 is (HUDAH) 11 HE 5 W 30 o 110 ·3H 2 O.
Embodiment 1-3
[0043] Example 1-3: DOHDA coated K 8 [Co 2 W 12 o 42 ](PM-3)
[0044] Dissolve 0.2 g of DOHDA in 20 mL of deionized water, 0.19 g of K 8 co 2 W 12 o 42 Dissolve in 20 ml deionized water, DOHDA and K 8 co 2 W 12 o 42 The molar ratio is 8:1 (the charge ratio is 8:8). Under stirring, will contain K 8 co 2 W 12 o 42 The aqueous solution of DOHDA was added dropwise to the aqueous solution of DOHDA. After the dropwise addition, continue to stir for 5 hours, filter the resulting precipitate with a melting sand funnel, obtain a green solid and dry it in vacuum at room temperature to obtain DOHDA-embedded K 8 [Co 2 W 12 o 42 ] Complex (abbreviated as SEP-3), the yield was 55%. SEP-3 elemental analysis: C% 34.65, H% 6.66, N% 1.70, the chemical formula of SEP-3 is (DOHDA) 7 co 2 W 12 o 42 4H 2 O.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com