Production and application of high stability recombinant trypsin
A trypsin and trypsinogen technology, which is applied in the production and application fields of recombinant trypsin, can solve the problems of low renaturation rate of inclusion bodies, inability to obtain effective expression of trypsin, and low yield of recombinant trypsin, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0068] Embodiment 1, the soluble expression of fusion protein (N-terminal 159 amino acids+human trypsinogen)
[0069] A polypeptide consisting of 159 amino acids is added to the N-terminus of human trypsinogen (GenBank: M27602.1, positions 17-247 (ie, excluding the signal peptide before the leader peptide), its sequence is (SEQ ID NO: 1 ):
[0070] MSDKIIHLTDDSFDTDVLKADGAILVDFWAEWCGPCKMIAPILDEIADEYQGKLTVAKLNIDQNPGTAPKYGIRGIPTLLLFKNGEVAATKVGALSKGQLKEFLDANLAGSGSGHMHHHHHHSSGLVRGSGMKETAAAKFERQHMDSPDLGTENLYFQS
[0071] Among them, there are 12 aspartic acid (D).
[0072] Design the following primers:
[0073] Forward: aa ggatcc AAAATCGTGGGTGGTTAC (SEQ ID NO: 6);
[0074] Reverse: cc AAGCTT TTAAGAGTTAGCAGCGA (SEQ ID NO: 7);
[0075] Using the human pancreas cDNA library as a template, the coding sequence of human trypsinogen was obtained by performing PCR amplification with the aforementioned primers.
[0076] The sequence obtained above was digested with HindIII / BamHI and ...
Embodiment 2
[0079] The purification of the fusion protein that embodiment 2, embodiment 1 obtain
[0080] The supernatant collected after the above-mentioned induced expression cells were sonicated was used as a sample solution to purify the recombinant fusion protein.
[0081] Use 2×15cm chromatography column, the ion exchange column is equilibrated with 20mM Tris-HCl, pH 8.0 buffer in advance, then the supernatant is loaded, equilibrated with the same buffer until the baseline is stable, then use 0-1M NaCl or 0~0.5M CaCl 2 Gradient elution, collection in sections, measure the OD280 and enzyme activity of each tube, combine the collected solutions with enzyme activity, and measure the total enzyme activity and total protein content. Combine the collection tubes with high enzyme activity to obtain purified active recombinant trypsin.
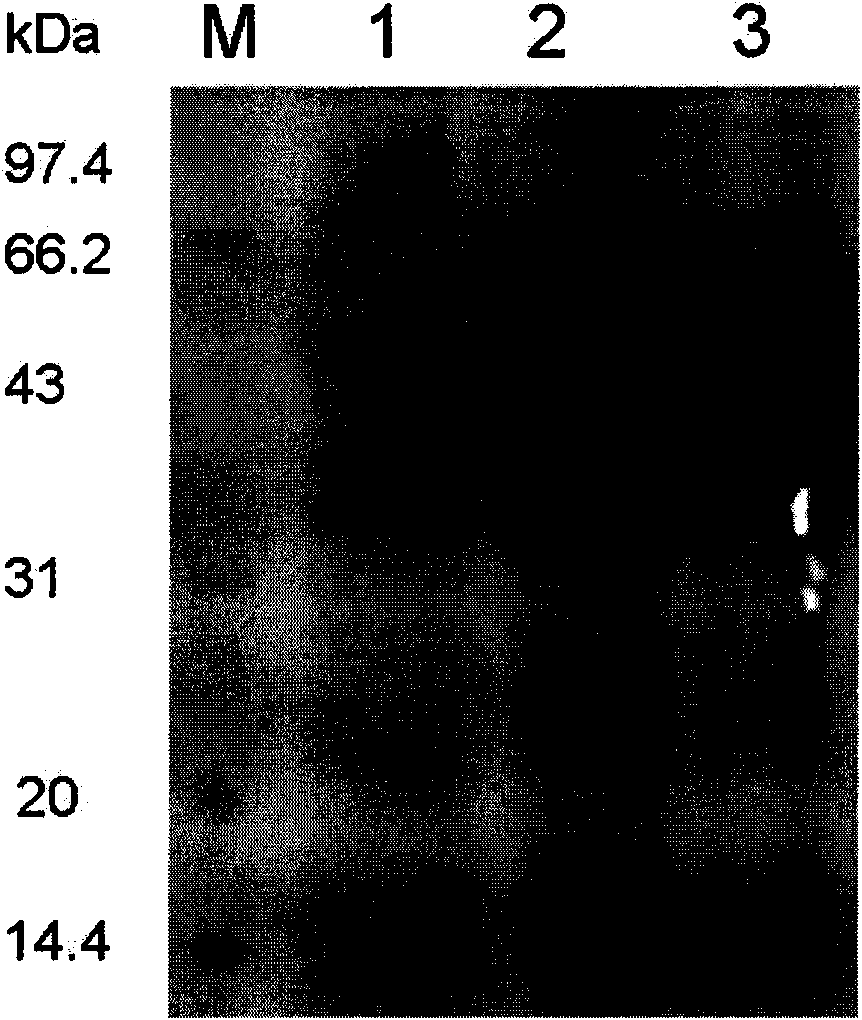
[0082] The collected purified solution was tested by SDS-PAGE for the purification results, see figure 2 . Among them, lane 1 is the loading solution;...
Embodiment 3
[0084] Embodiment 3, activation conditions
[0085] Using porcine pancreatic trypsin (purchased from Sigma Company), the fusion protein obtained from the aforementioned purification was activated by enzymatic hydrolysis to obtain active recombinant trypsin. Wherein, the amount of porcine pancreatic trypsin added is: porcine pancreatic trypsin:fusion protein (w:w)=1:1000. After activation, the recombinant trypsin with the activity of the amino acid fused at the N-terminus and the 7 amino acids at the N-terminus removed was obtained.
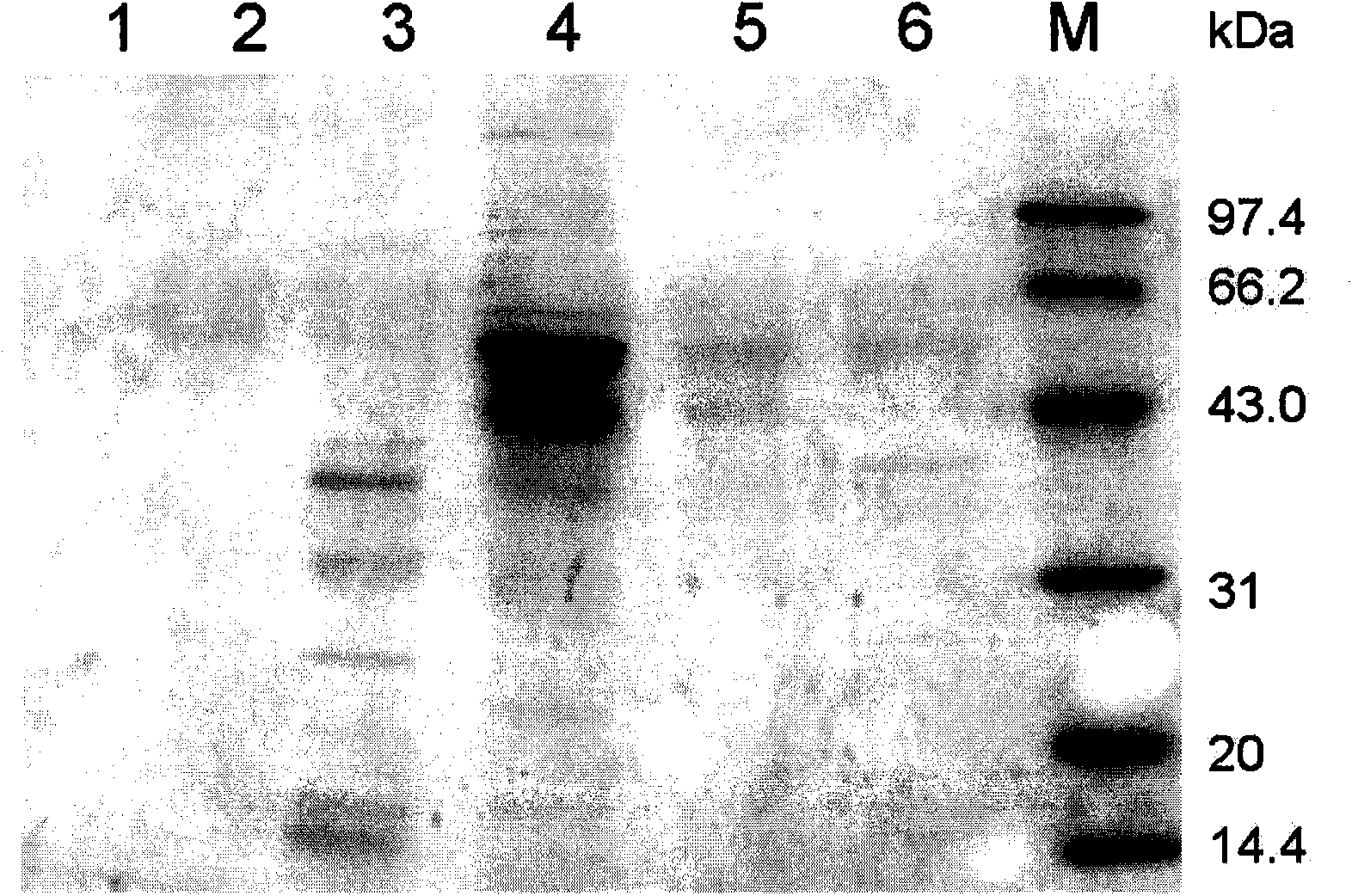
[0086] The enzymatic hydrolysis conditions are shown in Table 1.
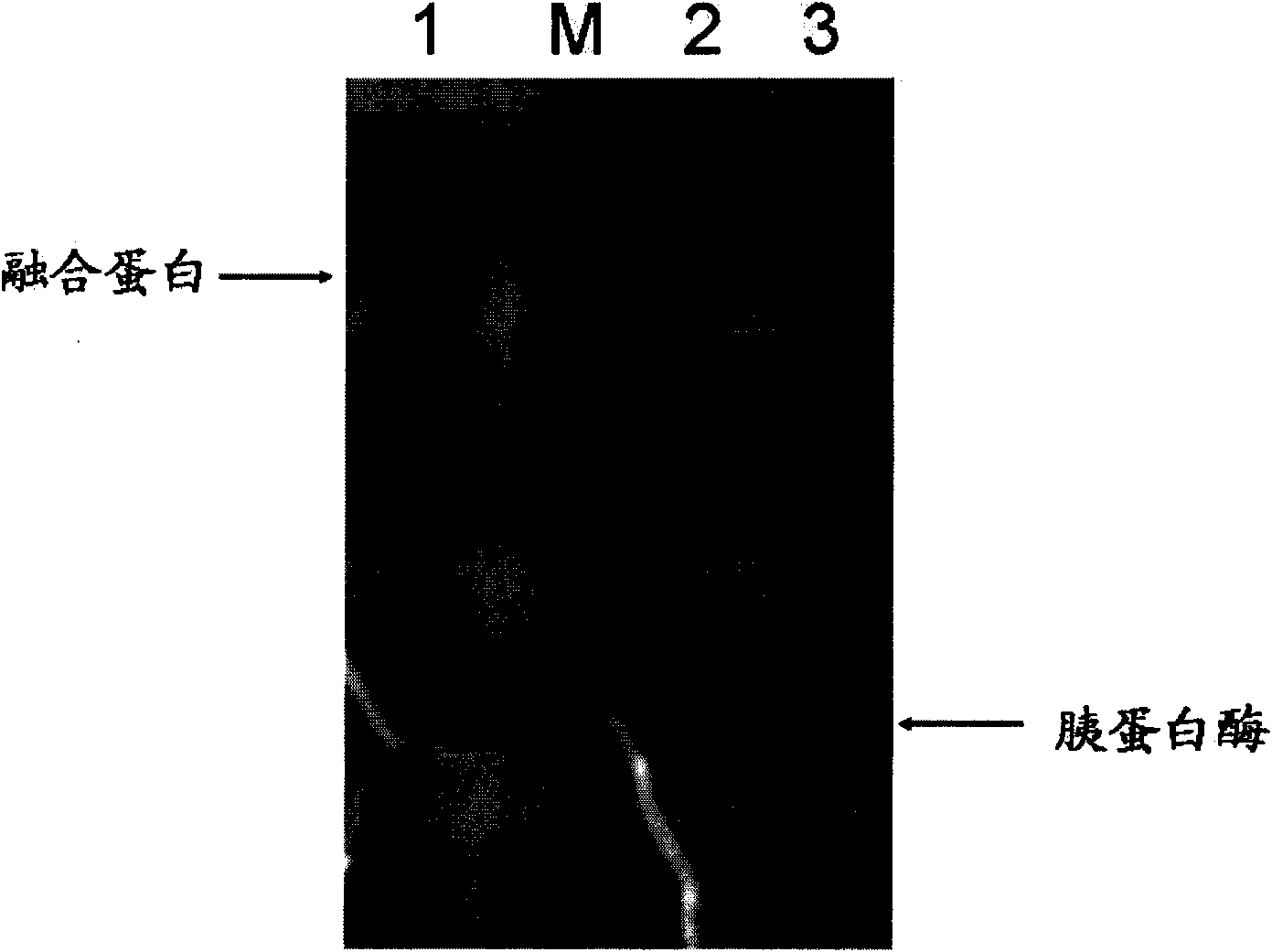
[0087] image 3 It is the electrophoresis identification result of the enzymolysis solution obtained after enzymolysis at 4°C for 25 hours. Among them, swimming lane 1 is the electrophoresis result of the purified enzyme solution (fusion protein); swimming lane 2 is the corresponding figure 2 Lane 3 is the electrophoresis result after enzymolysis; lane 3 is the electrophoresis...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com