Soluble expression system and application thereof in soluble expression of protein
An expression system and soluble technology, which is applied in the application field of soluble expression system and protein soluble expression, can solve the problems of complex operation and low renaturation rate, and achieve the effect of reducing costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0127] Embodiment 1, plasmid construction
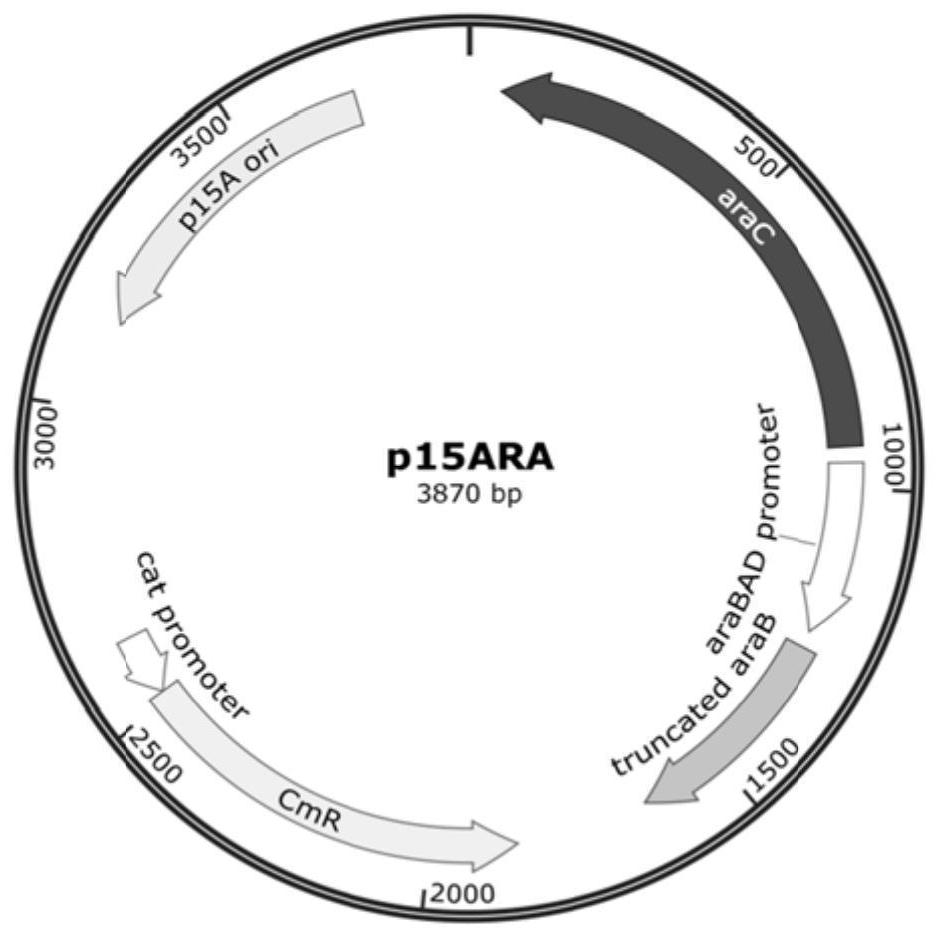
[0128] 1. Construction of plasmid p15ARA
[0129] araC-P BAD The sequence was derived from the genome sequence of E. coli BL21 (DE3) (NCBI VERSION: CP001509.3).
[0130] 1. Using the BL21(DE3) genome as a template and PARA-5 and PARA-3 as primers, about 1.7kb of PCR amplification containing araC gene, P BAD Promoter and DNA fragment ARA of partial araB gene sequence.
[0131] 2. Using plasmid pACYCDuet-1 as template, ACYC-5 and ACYC-3 as primers, PCR amplification containing Cm R and the DNA backbone ACYC of p15Aori (about 2.2 kb).
[0132] 3. In vitro recombination of backbone ACYC and fragment ARA to construct plasmid p15ARA (structural schematic diagram as shown in figure 1 shown). All in vitro recombination operations were completed using the recombination cloning kit of Nanjing Novizan Biotechnology Co., Ltd.
[0133] 4. Pick a single colony and use P15AC and ACYC-33 as primers for PCR identification. About 1.4kb is posit...
Embodiment 2
[0328] Embodiment 2, the construction of bacterial strain
[0329] 1. Construction of expression strains of sulfhydryl oxidase and protein disulfide bond isomerase
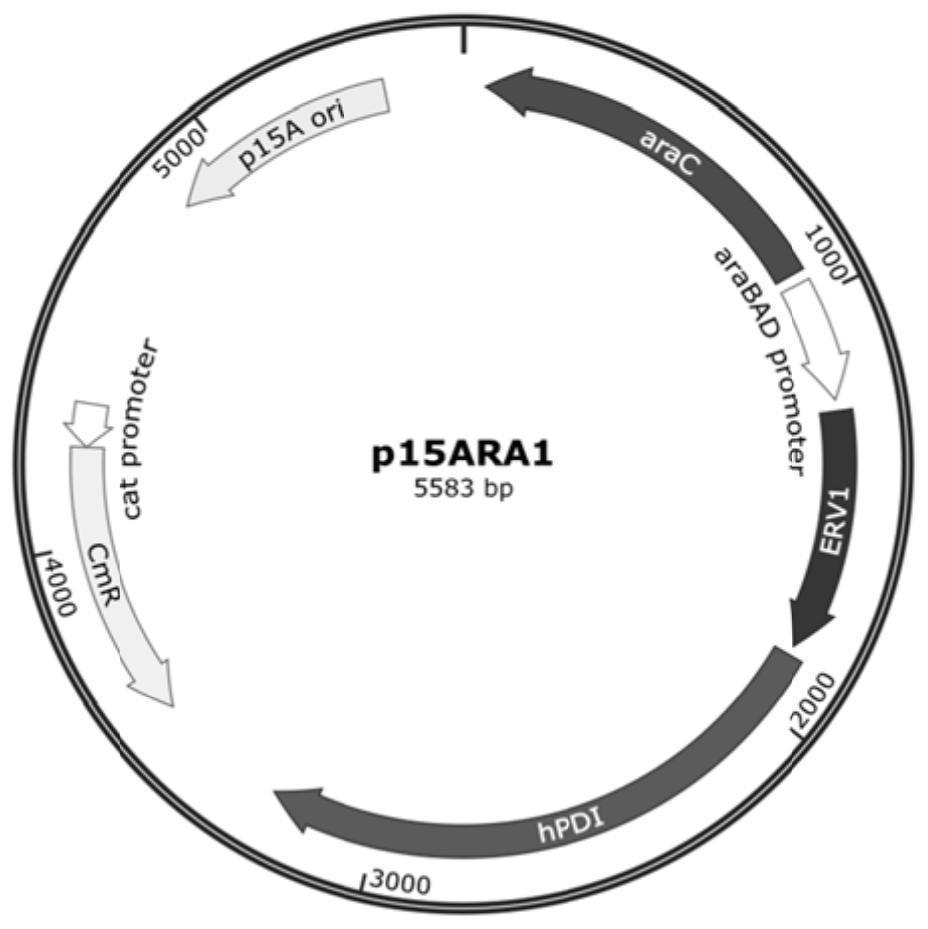
[0330] 1. The plasmid p15ARA1 was transformed into the strain BL21(DE3), and the strain BL21(DE3) / p15ARA1 was obtained, which was named WF1. This strain expresses ERV1 and hPDI.
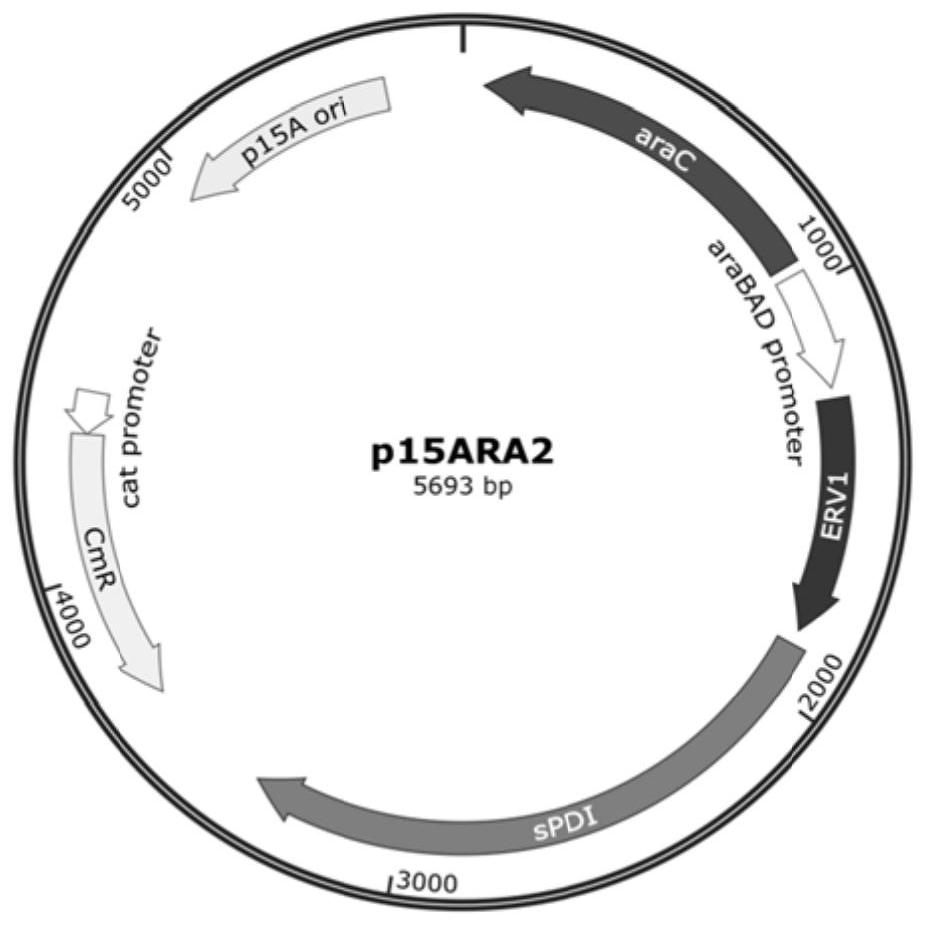
[0331] 2. The plasmid p15ARA2 was transformed into the strain BL21(DE3), and the strain BL21(DE3) / p15ARA2 was obtained, which was named WF2. This strain expresses ERV1 and sPDI.
[0332]3. The plasmid p15ARA3 was transformed into the strain BL21(DE3), and the strain BL21(DE3) / p15ARA3 was obtained, which was named WF3. This strain expresses ERV1 and DsbC.
[0333] 4. The plasmid p15T1 was transformed into the strain BL21(DE3), and the strain BL21(DE3) / p15T1 was obtained, which was named WF4. This strain expresses ERV1 and hPDI.
[0334] 5. The plasmid p15T2 was transformed into the strain BL21(DE3), and the strain BL21(DE3) / p15T2 wa...
Embodiment 3
[0377] Example 3, N-terminal tag optimization strategy to achieve soluble expression of sulfhydryl oxidase and protein disulfide bond isomerase
[0378] Culture strains BL21(DE3), WF1, WF2 and WF3, when OD 600nm Adding L-arabinose at 0.3–0.4 induces the expression of corresponding sulfhydryl oxidase (ERV1) and protein disulfide bond isomerase (hPDI, sPDI or DsbC), and the SDS-PAGE electrophoresis results of soluble protein and inclusion body protein are as follows Figure 43 shown. The protein molecular weights of ERV1, hPDI, sPDI and DsbC are about 21.70 kDa, 52.05 kDa, 55.70 kDa and 23.51 kDa, respectively. Compared with the control BL21(DE3), there is no band of the target protein.
[0379] The promoters of the sulfhydryl oxidase and protein disulfide isomerase genes were replaced with stronger T7 promoters. Cultivate strains BL21(DE3), WF4, WF5 or WF6, add inducer IPTG, express corresponding sulfhydryl oxidase (ERV1) and protein disulfide bond isomerase (hPDI, sPDI or D...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com