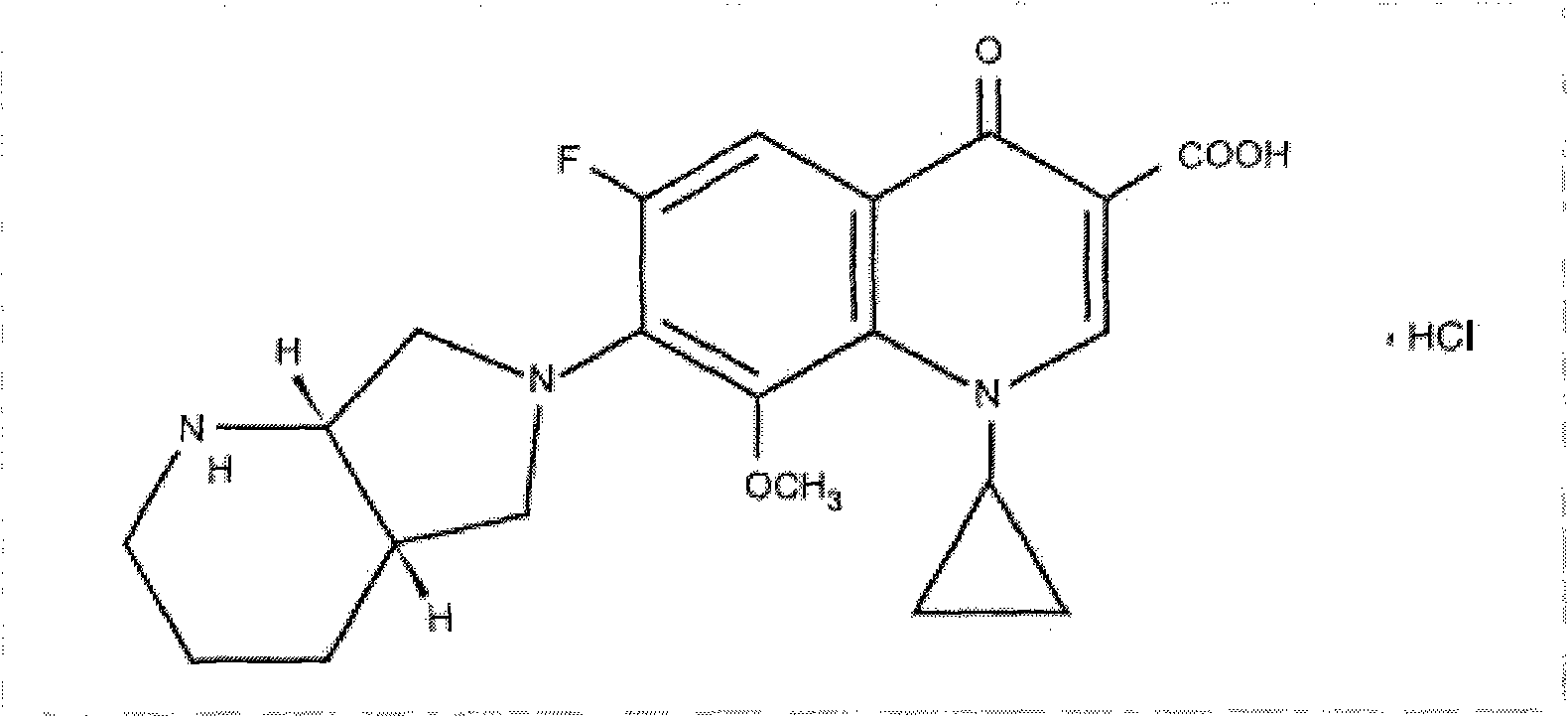
Synthesizing method of moxifloxacin hydrochloride
A technology of moxifloxacin hydrochloride and a synthesis method, applied in the field of medicinal chemistry, can solve the problems of difficulty in realizing industrialized production, cumbersome post-processing operations, product loss, etc., and achieve the effects of reducing operation steps, process optimization, and improving selectivity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0016] For a better description of the present invention, examples are as follows:
[0017] 1. Synthesis of quinoline carboxylic acid borane chelate
[0018] Take 86g of acetic anhydride and add it to a three-necked flask, add 3g of zinc chloride as a catalyst, stir at room temperature and slowly add 17.3g of boric acid to keep the temperature stable. After the addition, raise the temperature to 100°C for 3 hours. After the reaction was completed, the temperature was lowered to 70°C, and 83g (1-cyclopropyl-6,7-difluoro-1,4-dihydro-8-methoxy-4-oxo-3-quinolinecarboxylic acid) was added and stirred After 10 minutes, the temperature was raised to 100°C and the reaction was stirred for 3 hours. After the reaction was completed, the temperature was lowered to 40° C., then poured into 600 ml of frozen purified water and vigorously stirred. A large amount of solids precipitated, filtered, washed twice with ice water, dried and weighed: 89.5g.
[0019] 2. Synthesis of borane chelate...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com