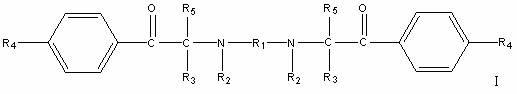
Macromolecular bifunctional amino ketone photoinitiator and preparation method thereof
A technology of group amino ketone and photoinitiator, which is applied in the field of macromolecular bifunctional amino ketone photoinitiator and its preparation, can solve the problems of less ineffective groups, molecular chain growth, poor compatibility, etc., and achieve wide application Foreground, lighten volatile, induce high-efficiency effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] N,N'-bis(1-phenylbenzyl-1-ethyl-2-(4-morpholinophenyl)ethanone)-N,N'-dimethyl-1,3-propanediamine ( Its chemical structural formula is the preparation of following formula II):
[0040] Step 1: Add 24.5g of 1-(4-fluorophenyl)-2-bromobutanone, 100mL of 1,2-dichloroethane, 13.8 g potassium carbonate, drop the temperature to 20°C and add 5.1g N,N'-dimethyl-1,3-propanediamine dropwise, continue the reaction for 10h after the drop, after the reaction, the organic phase was washed twice with 20mL water, and separated The aqueous phase was dried with anhydrous calcium chloride, filtered, and the solvent was evaporated to obtain N,N'-bis(1-ethyl-2-(4-fluorophenyl)ethanone)-N,N'-dimethyl - 1,3-propanediamine 21 g.
[0041] Step 2: In the 250mL four-necked flask that condenser is housed, thermometer, add 21g last step product, 100g morpholine, 13g anhydrous potassium carbonate in the 250mL four-neck flask of agitator, reflux reaction until no raw material, decompression di...
Embodiment 2
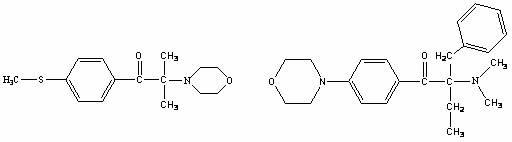
[0046] N,N'-bis(1,1-dimethyl-2-(4-methylthiophenyl)ethanone)-N,N'-dimethyl-1,3-propanediamine (its chemical formula For the preparation of formula III) below:
[0047]
[0048] Step 1: Add 27.3g of 1-(4-methylthiophenyl)-2-methyl-2-bromoacetone, 100mL of 1,2-di Ethyl chloride, 13.8g of potassium carbonate, 5.1g of N,N'-dimethyl-1,3-propanediamine was added dropwise at room temperature, and the reaction was continued for 6h after the drop was completed. The water phase was removed, and the organic phase was dried with anhydrous calcium chloride, filtered and evaporated to remove the solvent.
[0049] Step 2: Add 50 mL of ethanol to the product in the previous step, heat and stir to reflux until the system is clear, then filter while hot, the solution is cooled to crystallize, suction filtered, and dried. Obtain 22g of white powder product, yield 90%.
Embodiment 3
[0051] N,N'-bis(1,1-dimethyl-2-(4-phenylphenyl)ethanone)-N,N'-dimethyl-1,3-propanediamine (its chemical formula is Preparation of formula IV) below:
[0052]
[0053] Step 1: Add 30.3g of 1-(4-phenylphenyl)-2-methyl-2-bromoacetone, 100mL of 1,2-di Ethyl chloride, 13.8g of potassium carbonate, 5.1g of N,N'-dimethyl-1,3-propanediamine was added dropwise at room temperature, and the reaction was continued for 5h after the drop was completed. The water phase was removed, and the organic phase was dried with anhydrous calcium chloride, filtered and evaporated to remove the solvent.
[0054] Step 2: Add 50 mL of ethanol to the product in the previous step, heat and stir to reflux until the system is clear, then filter while hot, the solution is cooled to crystallize, suction filtered, and dried. Obtained 25.1 g of white powder product, yield 92%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com