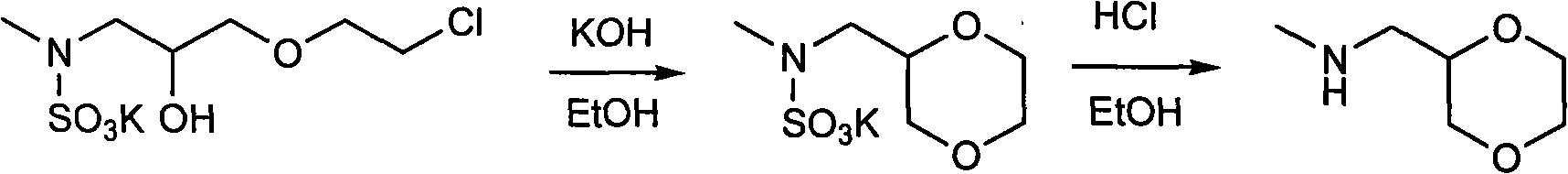
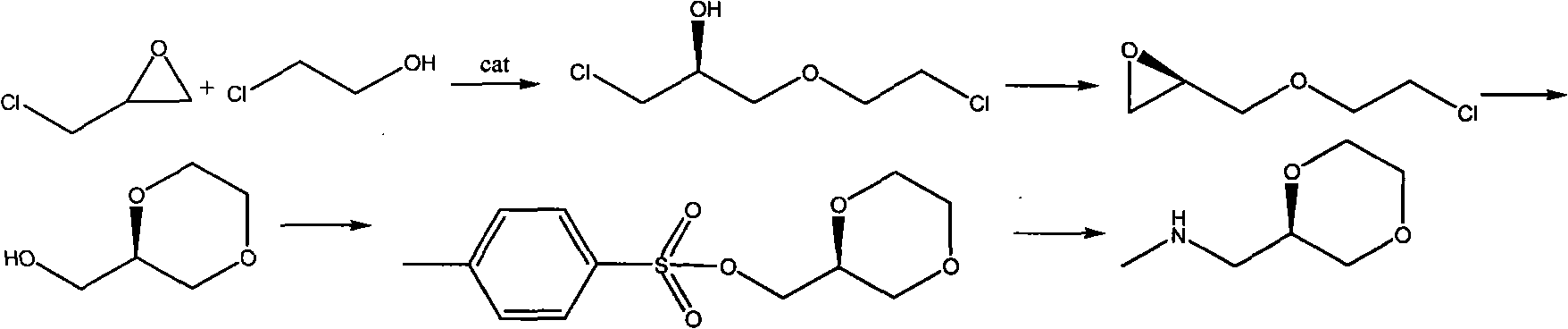
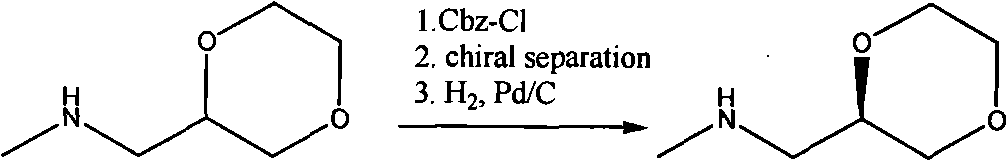Synthesis process of (2R)-(1,4-dioxane-2-yl)-N-methyl-methanamine hydrochloride
A technology of methyl methylamine hydrochloride and synthesis process, applied in directions such as organic chemistry, can solve problems such as high cost of chiral column, bringing in by-products, difficulty in industrialization, etc., avoiding chiral splitting, simple raw materials, The effect of easy availability of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] 1) Add 0.05 grams of [(R, R)-N, N'-bis(3,5-di-tert-butyl salicylidene)-1,2-cyclohexanediamine cobalt in a four-necked flask 2 ·SnCl 2 100 grams of epichlorohydrin and 50 grams of 2-chloroethanol were reacted at 0°C for 12 hours, cooled to 10°C, added 500 grams of dichloromethane, neutralized to pH= 7.0-8.0, standing to separate layers, drying the organic layer with anhydrous sodium sulfate, distilling off dichloromethane to obtain crude product 81.3 of (S)-3-(2-chloroethoxy)-1-chloropropan-2-ol gram, gas chromatography analysis, content 93.7%, directly used in next step reaction;
[0020] 2) Add 250 grams of acetone in a four-necked flask, 50 grams of (S)-3-(2-chloroethoxy)-1-chloropropan-2-alcohol and 50 grams of sodium carbonate reflux reaction for 3 hours, cooled to 0 ℃, filter, remove insoluble matter, distill off acetone, obtain (2R)-2-[(2-chloroethoxy) methyl] oxirane crude product 28.9 grams, gas chromatography analysis, content 91.6%, directly for the next re...
Embodiment 2
[0024] 1) Add 0.05 grams of [(R, R)-N, N'-bis(3,5-di-tert-butyl salicylidene)-1,2-cyclohexanediamine cobalt in a four-necked flask 2 ·SnCl 4 100 grams of epichlorohydrin and 100 grams of 2-chloroethanol were reacted at 20°C for 24 hours, cooled to 20°C, added 500 grams of dichloromethane, neutralized to pH= 7.0-8.0, standing to separate layers, the organic layer was dried with anhydrous sodium sulfate, dichloromethane was evaporated to obtain (S)-3-(2-chloroethoxy)-1-chloropropan-2-ol crude product 70.5 gram, gas chromatography analysis, content 93.5%, directly used in next step reaction;
[0025] 2) Add 250 grams of acetone in a four-necked flask, 50 grams of (S)-3-(2-chloroethoxy)-1-chloropropane-2-alcohol and 150 grams of sodium carbonate reflux reaction for 5 hours, cooled to 10 DEG C, filter, remove insoluble matter, evaporate acetone, obtain (2R)-2-[(2-chloroethoxy) methyl] oxirane crude product 28.1 grams, gas chromatography analysis, content 91.6%, directly for the ...
Embodiment 3
[0029] 1) Add 0.05 grams of [(R, R)-N, N'-bis(3,5-di-tert-butyl salicylidene)-1,2-cyclohexanediamine cobalt in a four-necked flask 2 ·FeCl 3 100 grams of epichlorohydrin and 75 grams of 2-chloroethanol were reacted at 15°C for 20 hours, cooled to 15°C, added 500 grams of dichloromethane, neutralized to pH= 7.0-8.0, standing to separate layers, drying the organic layer with anhydrous sodium sulfate, evaporating dichloromethane to obtain (S)-3-(2-chloroethoxy)-1-chloropropan-2-ol crude product 80.0 gram, gas chromatography analysis, content 93.7%, directly used in next step reaction;
[0030] 2) Add 250 grams of acetone in a four-necked flask, 50 grams of (S)-3-(2-chloroethoxy)-1-chloropropan-2-alcohol and 75 grams of potassium carbonate reflux reaction for 4 hours, cooled to 0 ℃, filter, remove insoluble matter, evaporate acetone, obtain (2R)-2-[(2-chloroethoxy) methyl] oxirane crude product 29.1 grams, gas chromatography analysis, content 91.5%, directly for the next reacti...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com