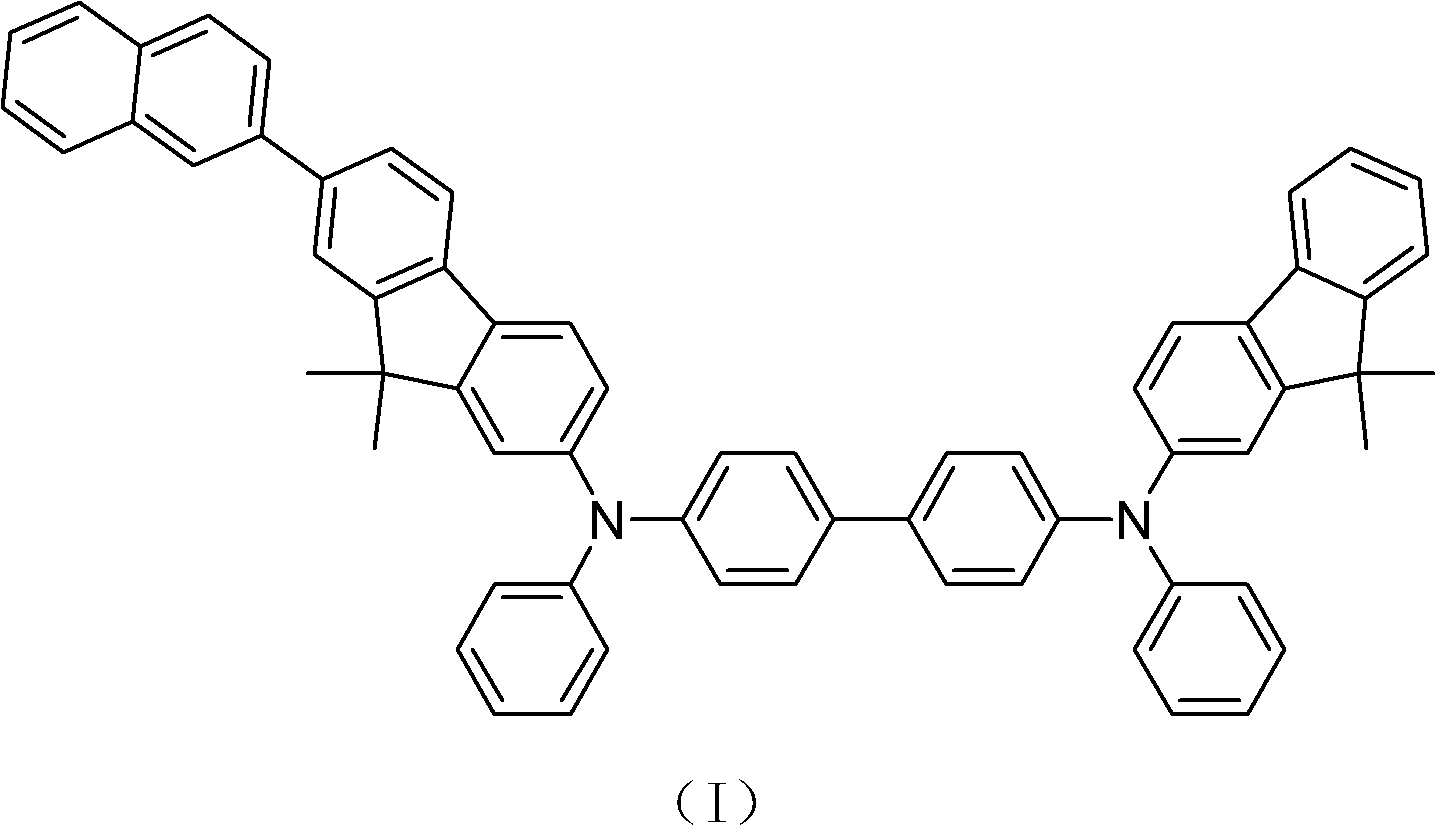
N, N'-diphenyl-N-(9, 9-dimethyl-2-fluorenyl)-N'-(9, 9-dimethyl-7'-(2''-naphthyl)-2'-fluorenyl)-benzidine and synthesis method thereof
A synthesis method and dimethyl technology, applied in the field of chemical synthesis of hole transport materials, can solve problems such as no effective methods have been found yet
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] 1) Add 500 grams of 9,9-dimethyl-2-aminofluorene to a 5-liter four-necked bottle, and then add 487.56 grams of iodobenzene, 1500 grams of toluene, 4.56 grams of cuprous iodide, and 4.32 grams of phenanthrene phylloline and 500 grams of potassium carbonate. The above reaction solution was stirred under reflux for 6 hours, cooled to room temperature, filtered, and the filtrate was washed with water until the pH value of the aqueous phase was 7-8. The filtrate organic phase after washing is separated from the aqueous phase, the aqueous phase is discarded, and the organic phase is retained, and then the above-mentioned organic phase is concentrated to dryness using a rotary evaporator to obtain a crude product, and finally the crude product is subjected to silica gel column chromatography (the weight ratio of the crude product to silica gel is 1:10) and ethyl acetate / petroleum ether (the weight ratio of the two is 1:80) to obtain 388 grams of intermediate 1 (9,9-dimethyl-2-...
Embodiment 2
[0050] 1) 83 grams of 9,9-dimethyl-2-aminofluorene are added to a four-necked flask, and then 50 grams of iodobenzene, 300 grams of toluene, 0.92 grams of cuprous iodide, 0.87 grams of phenanthroline and 120 grams of potassium carbonate. The reaction solution was stirred under reflux for 6 hours, cooled to room temperature, filtered, and the filtrate was washed with water until the pH value of the aqueous phase was 7-8. The filtrate organic phase after washing is separated from the aqueous phase, the aqueous phase is discarded, and the organic phase is retained, and then the above-mentioned organic phase is concentrated to dryness using a rotary evaporator to obtain a crude product, and finally the crude product is subjected to silica gel column chromatography (the weight ratio of the crude product to silica gel is 1:8) and ethyl acetate / petroleum ether (the weight ratio of the two is 1:50) to obtain 46 grams of intermediate 1 (9,9-dimethyl-2-(N-phenyl)- Fluorene), the yield ...
Embodiment 3
[0059] 1) 70 grams of 9,9-dimethyl-2-aminofluorene are added to a four-necked flask, and then 35 grams of iodobenzene, 350 grams of toluene, 0.77 grams of cuprous iodide, 0.73 grams of phenanthroline and 130 grams of potassium carbonate. The reaction solution was stirred under reflux for 6 hours. Cool to room temperature, filter, and wash the filtrate with water until the pH of the aqueous phase is 7-8. The filtrate organic phase after washing is separated from the aqueous phase, the aqueous phase is discarded, and the organic phase is retained, and then the above-mentioned organic phase is concentrated to dryness using a rotary evaporator to obtain a crude product, and finally the crude product is subjected to silica gel column chromatography (the weight ratio of the crude product to silica gel is 1:6) and ethyl acetate / petroleum ether (the weight ratio of the two is 1:70) to obtain 36 grams of intermediate 1 (9,9-dimethyl-2-(N-phenyl)- Fluorene), the yield is 73%.
[0060...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com