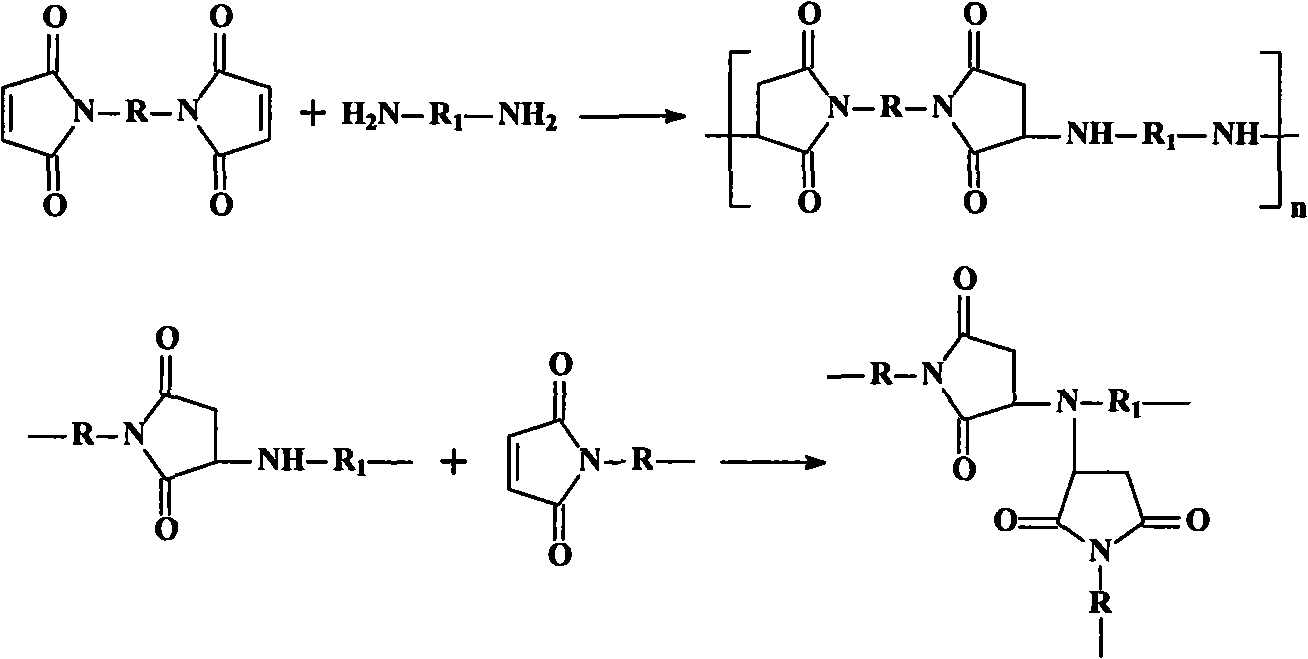
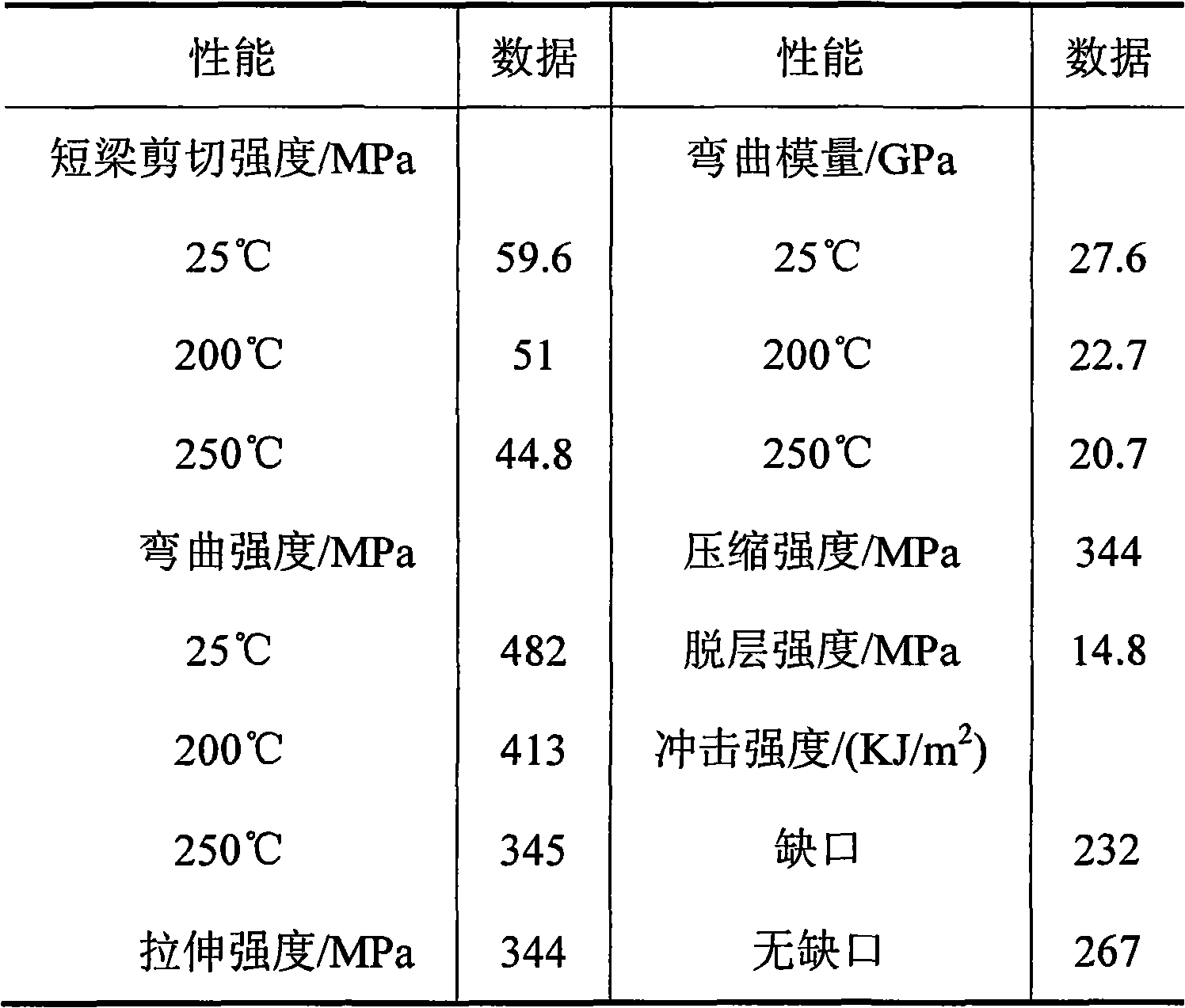
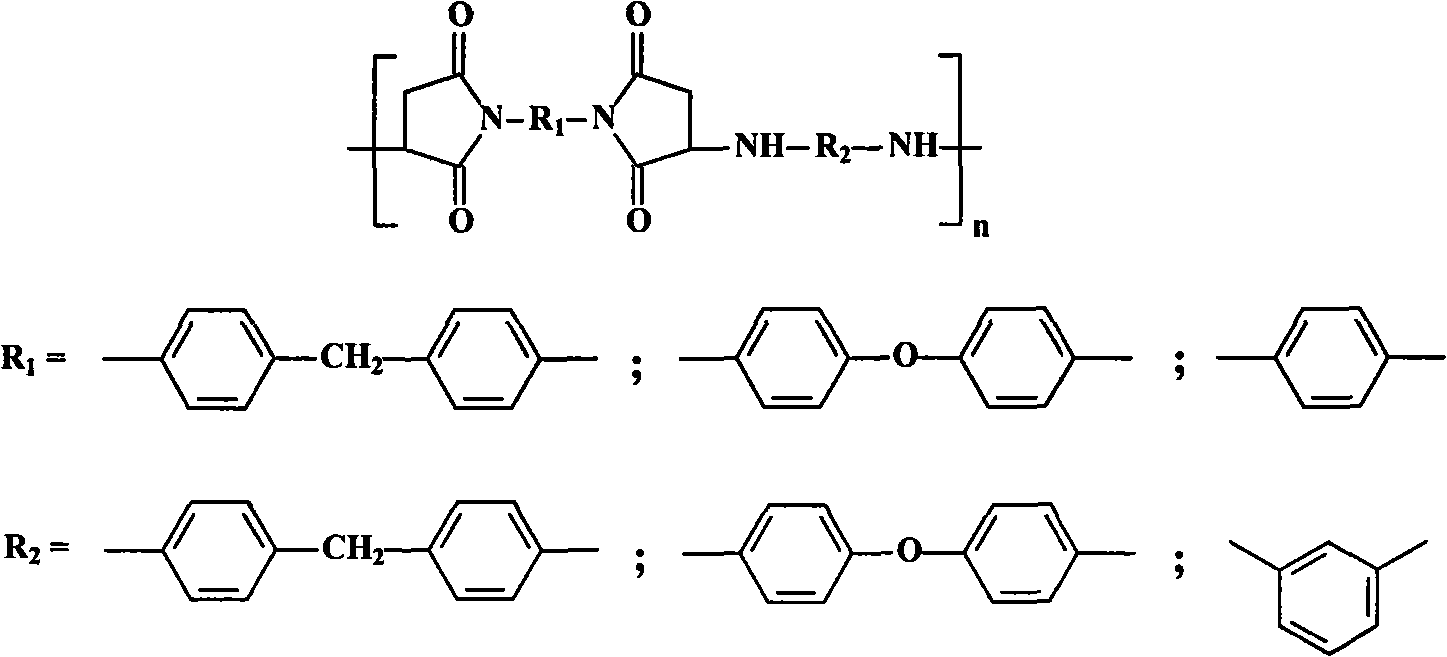
Triazole diamine-containing modified bismaleimide resin and preparation method thereof
A bismaleimide resin, bismaleimide technology, applied in the direction of organic chemistry, etc., can solve the problems of high molding temperature, difficult preparation of composite materials, poor viscosity of prepregs, etc. Good processability, low moisture absorption effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] (1) Synthesis of 1,4-diazidemethylbenzene
[0050] Add p-dichloromethylbenzene (0.05mol), NaN 3 (0.15mol), toluene (20mL) and N,N-dimethylformamide (20mL), heated to 70~75°C under stirring, and reacted at constant temperature for 3h. After the reaction, the reaction product was cooled to room temperature and poured into 200mL In deionized water, stand overnight under freezing conditions to precipitate white flaky crystals, filter, wash the filter cake with deionized water, and dry to obtain a white powdery solid with a yield of 90% and a melting point of 27-29°C; FT-IR (KBr, v, cm -1 ): 2089 (-N 3 stretching vibration); 1 H-NMR (CDCl 3 , TMS) δ[subscript indicates structure position]: 7.33(s, 4H, H a ), 4.35(s, 4H, H b ), its structural formula is:
[0051]
[0052] (2) Synthesis of PMDTA
[0053] Add 1,4-diazidemethylbenzene (0.01mol), m-aminophenylacetylene (0.02mol), CuSO 4 ·5H 2 O (0.005mol), sodium ascorbate (0.01mol), N,N-dimethylformamide (25mL), sti...
Embodiment 2
[0060] Preparation of BMI-PMDTA-2
[0061] Add 3.58g (0.01mol) of BMI, 2.72g (0.006mol) of PMDTA and 15mL of DMF into a 100mL three-necked flask, mechanically stir, pass condensed water, N 2 Protected and reacted at 70°C for 2h. After the reaction, cool to room temperature, pour into 1000mL deionized water to precipitate, separate the precipitate by suction filtration, and vacuum dry to obtain the product as a gray powdery solid with a yield of 90% and a melting point of 142°C. BMI-PMDTA-2 can be dissolved in solvents such as DMF, DMAc and NMP. FT-IR (KBr, v, cm -1 ): 3462(-NH-), 3420, 3339 and 1616(-NH 2 ), 3134 (triazole ring-H), 1704 (C=O), 1147 (C-N-C), 1177 (C-N-C after the double bond on the imide ring is opened), its structural formula is:
[0062]
[0063] in
[0064]
[0065] The resin was cured in an oven according to the program 160°C / 2h+180°C / 2h+200°C / 2h+240°C / 5h to obtain a dense and hard dark brown cured product. T measured by DSC analysis (20°C / min...
Embodiment 3
[0067] Preparation of BMI-PMDTA-3
[0068] Add 3.58g (0.01mol) of BMI, 3.62g (0.008mol) of PMDTA and 15mL of DMF into a 100mL three-necked flask, mechanically stir, pass condensed water, N 2 Protected and reacted at 70°C for 2h. After the reaction, cool to room temperature, pour into 1000mL deionized water for precipitation, separate the precipitate by suction filtration, and vacuum dry to obtain the product as a gray powdery solid with a yield of 90% and a melting point of 148°C. BMI-PMDTA-3 can be dissolved in solvents such as DMF, DMAc and NMP. FT-IR (KBr, v, cm -1 ): 3462(-NH-), 3420, 3339 and 1616(-NH 2 ), 3134 (triazole ring-H), 1704 (C=O), 1147 (C-N-C), 1177 (C-N-C after the double bond on the imide ring is opened), its structural formula is:
[0069]
[0070] in
[0071]
[0072] The resin was cured in an oven according to the program 160°C / 2h+180°C / 2h+200°C / 2h+240°C / 5h to obtain a dense and hard dark brown cured product. T measured by DSC analysis (20°C / ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| glass transition temperature | aaaaa | aaaaa |
| decomposition temperature | aaaaa | aaaaa |
| yield stress | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com