Flavone analog, preparation and application thereof as anti-diabetic medicament
A technology of analogs and flavonoids, applied in the field of medicinal chemistry, can solve the problems of low efficacy, large dose, and little clinical significance.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
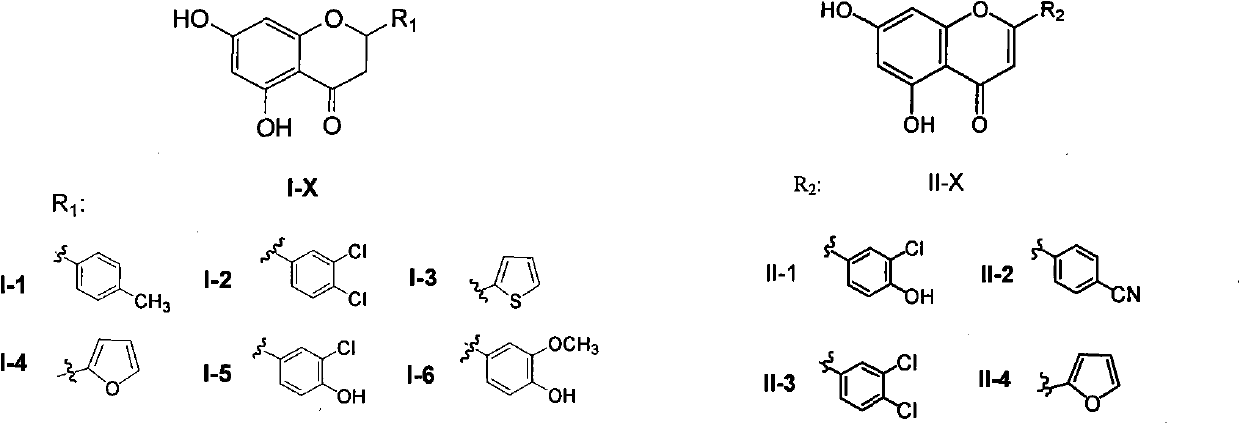
[0032] Synthetic General Method of Compound I-Xa
[0033]Synthesis of 2-Hydroxy-4,6-bis(methoxymethoxy)acetophenone
[0034] Weigh an appropriate amount of 2,4,6-trihydroxyacetophenone and dissolve it with an appropriate amount of anhydrous acetone, add 7 equivalents of potassium carbonate and stir, add 2.2 equivalents of chloromethyl methyl ether (MOMCl) dropwise with a dropping funnel, heat to reflux, and TLC Monitor until the reaction is complete. The reaction solution was cooled to room temperature and filtered, and the filtrate was concentrated under reduced pressure to obtain a crude product, which was subjected to flash silica gel column chromatography to obtain pure product 2-hydroxy-4,6-bis(methoxymethoxy)acetophenone.
[0035] Synthesis of Substituted Chalcone Derivatives I-Xa
[0036] Weigh an appropriate amount of 2-hydroxy-4,6-bis(methoxymethoxy)acetophenone into a reaction flask, and add an appropriate amount of methanol to dissolve it. Add 30 equivalents of 6...
Embodiment 2
[0039] Synthetic General Method of Series Compounds I-X
[0040] Synthesis of Compounds I-Xb
[0041] Weigh an appropriate amount of the corresponding chalcone compound I-Xa and dissolve it in an appropriate amount of ethanol, add 4.5 equivalents of sodium acetate, dropwise add 1-3 drops of distilled water, heat to reflux, and monitor by TLC until the product point intensity does not change. The reaction solution was cooled to room temperature and poured into an appropriate amount of water, extracted three times with ethyl acetate, combined organic phases, dried over anhydrous magnesium sulfate, filtered, and concentrated to obtain a crude product, which was subjected to flash silica gel column chromatography to obtain pure product I-Xb.
[0042] Synthesis of Compounds I-X
[0043] Weigh an appropriate amount of hydroxyl-protected dihydroflavone compounds I-Xb, dissolve them in an appropriate amount of methanol, add 1.5 equivalents of 3mol / l hydrochloric acid, stir and heat t...
Embodiment 3
[0058] Synthesis of 2-(3-chloro-4-hydroxyphenyl)-5,7-dihydroxybenzopyran-4-one (II-1)
[0059] Weigh an appropriate amount of the corresponding chalcone compound I-Xa, dissolve it with an appropriate amount of anhydrous pyridine, add 0.1 equivalent of solid iodine, stir and heat to reflux, and monitor by TLC until the reaction is complete. After the reaction solution was cooled to room temperature, an appropriate amount of ethyl acetate was added, washed three times with saturated sodium chloride solution, the organic phase was dried over anhydrous magnesium sulfate, filtered, and concentrated under reduced pressure to obtain crude product II-Xa.
[0060] Weigh an appropriate amount of the crude product II-Xa obtained in the previous step, dissolve it in an appropriate amount of methanol, add 15 equivalents of 3 mol / l hydrochloric acid, stir and heat to reflux, and monitor by TLC until the reaction is complete. The reaction solution was poured into ice water, extracted three t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com