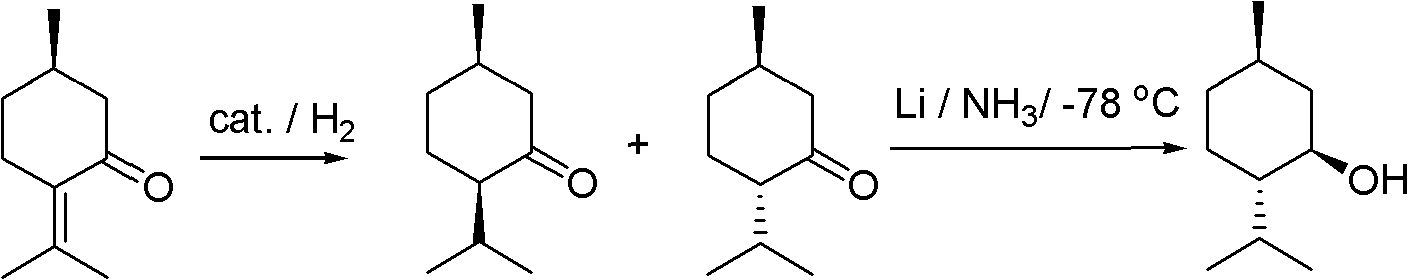
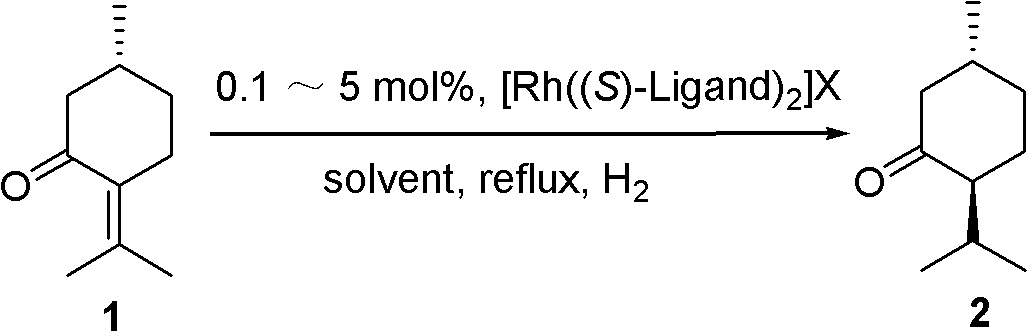Method for chiral synthesis of levorotatory menthol
A technology for chiral synthesis of L-menthol, applied in chemical instruments and methods, preparation of organic compounds, preparation of hydroxyl compounds, etc., can solve the problems of complex and harsh processes, low overall yield, poor selectivity, etc., and achieve Good selectivity, simple recovery and simple synthesis process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] 1. Preparation of catalyst
[0036] (1) Catalyst precursor [Rh(COD)Cl] 2 Synthesis
[0037] Add rhodium trichloride trihydrate (2.0g, 7.6mmol) into a 150mL three-necked flask. After putting in a magnetic stir bar, add 95% ethanol (30mL), water (12mL) and 1,5- Cyclooctadiene (6mL, 49mmol), stirred and refluxed for 24h, formed orange-yellow precipitate, cooled to room temperature, filtered, the product was washed with cold methanol-water mixture to remove unreacted 1,5-cyclooctadiene, and then used A little cold ether was used to infiltrate the solid, and then vacuum dried at 25°C for 8 hours. Remove the solvent to obtain orange-yellow crystals [Rh(COD)Cl] 2 (1.593g, yield 85%) m.p. 243°C.
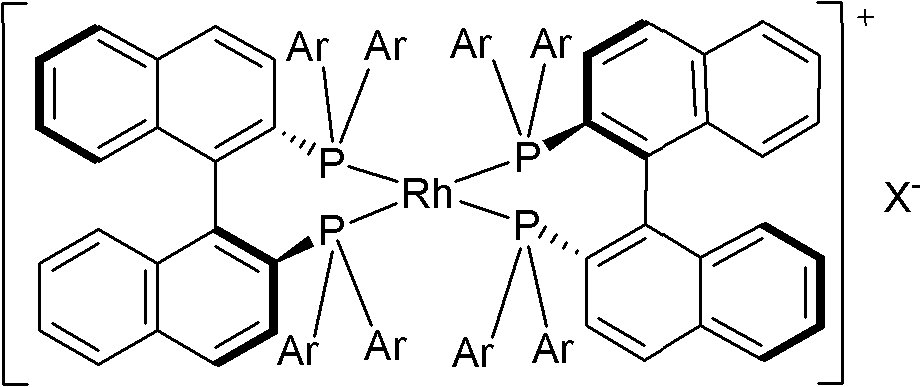
[0038] (2) Catalyst [Rh((S)-p-Tol-BINAP) 2 ]BF 4 Preparation
[0039] Under the protection of nitrogen, add 0.123g (0.25mmol) [Rh(COD)Cl] to a 25mL round bottom flask 2 And 0.339g(0.5mmol)(S)-p-Tol-BINAP, add 5.0mL freshly steamed CH 2 Cl 2 Dissolve the solid, put in a magnetic stir bar, and...
Embodiment 2
[0047] 1. Preparation of catalyst
[0048] (1) Catalyst precursor [Rh(COD)Cl] 2 Synthesis
[0049] Add rhodium trichloride trihydrate (1.0g, 3.8mmol) and anhydrous potassium carbonate (0.55g, 4mmol) into a 100mL three-necked flask, add 95% ethanol (17mL) and water (8.5mL) under nitrogen protection And 1,5-cyclooctadiene (3mL, 24.5mmol), put in a magnetic stirrer, stir at reflux for 24h, produce orange-yellow precipitate, cool to room temperature, filter, the product is washed with cold methanol-water mixture to remove unreacted 1,5-cyclooctadiene, and finally infiltrate the solid with a little cold ether, and then vacuum dry at 25℃ for 8h, remove the solvent, and obtain an orange-yellow product [Rh(COD)Cl] 2 (0.778g, yield 83%) m.p. 242°C.
[0050] (2) Catalyst [Rh((S)-BINAP) 2 ]BF 4 Preparation
[0051] Under the protection of nitrogen, add 0.15g (0.3mmol) [Rh(COD)Cl] to a 10mL round bottom flask 2 , 3mL freshly distilled tetrahydrofuran and 70% 2.5mL HBF 4 , Add 1,5-cyclooctadiene ...
Embodiment 3
[0059] 1. Preparation of catalyst
[0060] (1) Catalyst precursor [Rh(COD)Cl] 2 Synthesis
[0061] Add rhodium trichloride trihydrate (0.5g, 1.9mmol) into a 50mL three-necked flask, add 95% methanol (9mL), water (4mL) and 1,5-cyclooctadiene (0.14g) under nitrogen , 122mmol), put in a magnetic stirrer, stirred and refluxed for 24h to produce orange-yellow precipitate, cooled to room temperature, filtered, the product was washed with cold methanol-water mixture to remove unreacted 1,5-cyclooctadiene, and finally used A little cold ether soaks the solid, then vacuum-drys at 25℃ for 8h, removes the solvent, and recrystallizes with dichloromethane / ether to obtain an orange-yellow product [Rh(COD)Cl] 2 (0.393g, yield 84%) m.p. 243°C.
[0062] (2) Catalyst [Rh((S)-XylBINAP) 2 ]BF 4 Preparation
[0063] Under the protection of nitrogen, add 0.47g (0.95mmol) [Rh(COD)Cl] to a 10mL round bottom flask 2 Add 0.209g (1.9mmol) NaBF 4 , Put in a magnetic stir bar, add 3mL of tetrahydrofuran to disso...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com