Analytical reagent for heavy metal measurement as well as preparation method and application thereof
An analytical reagent and diazotization technology, which is applied in the field of analytical chemistry, can solve problems such as low sensitivity, practical application limitations, and insufficient stability of the generated complex, and achieve the effects of improved detection sensitivity, improved sensitivity, and reliable measurement results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] - Synthesis of analytical reagents
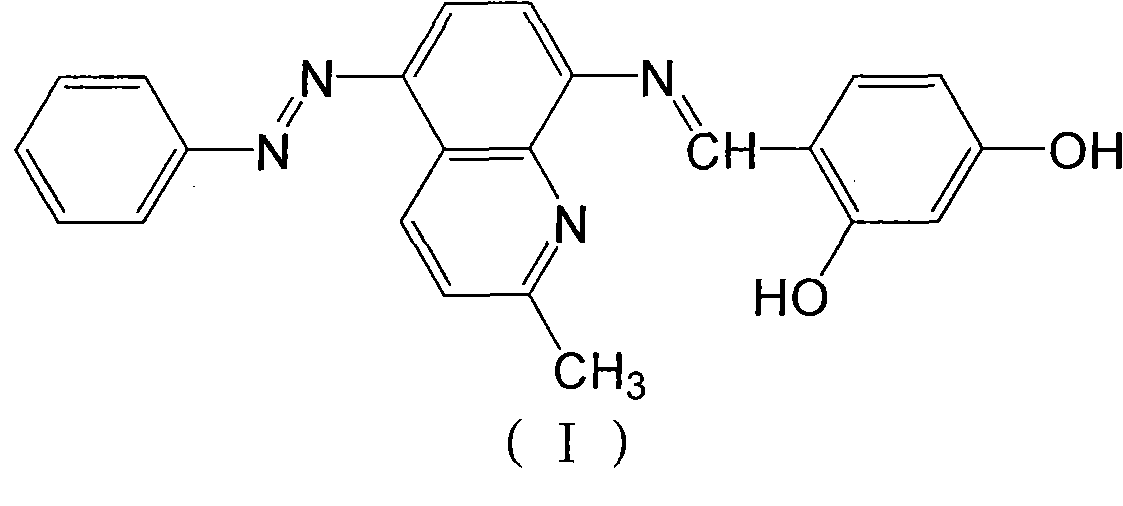
[0034] (1) Diazotization——mix 0.1 mol of aniline, 2.5 mL of concentrated hydrochloric acid and 3 mL of water, heat to dissolve, cool to below 5°C in an ice bath, and add 10 mL of 10% sodium nitrite aqueous solution dropwise under low temperature stirring. Continue to react for 30 minutes, add a small amount of urea to remove unreacted sodium nitrite. Dissolve 0.1moL of 8-aminoquinaridine in 20mL of 1:1 acetic acid, drop the above-mentioned diazonium salt into it under low temperature stirring, and add dilute sodium hydroxide at any time to control the pH value between 5-6, After coupling at low temperature for 2 hours, the crude product was obtained by filtration, and the low melting point part of the product was removed by sublimation to obtain a pure yellow product (5-phenylazo-8-aminoquinaridine), with a yield of 72%.
[0035] (2) Condensation——Dissolve 0.05mol of 5-phenylazo-8-aminoquinaridine in 6mL of absolute ethanol, heat to d...
Embodiment 2
[0037] - Chromatographic conditions used
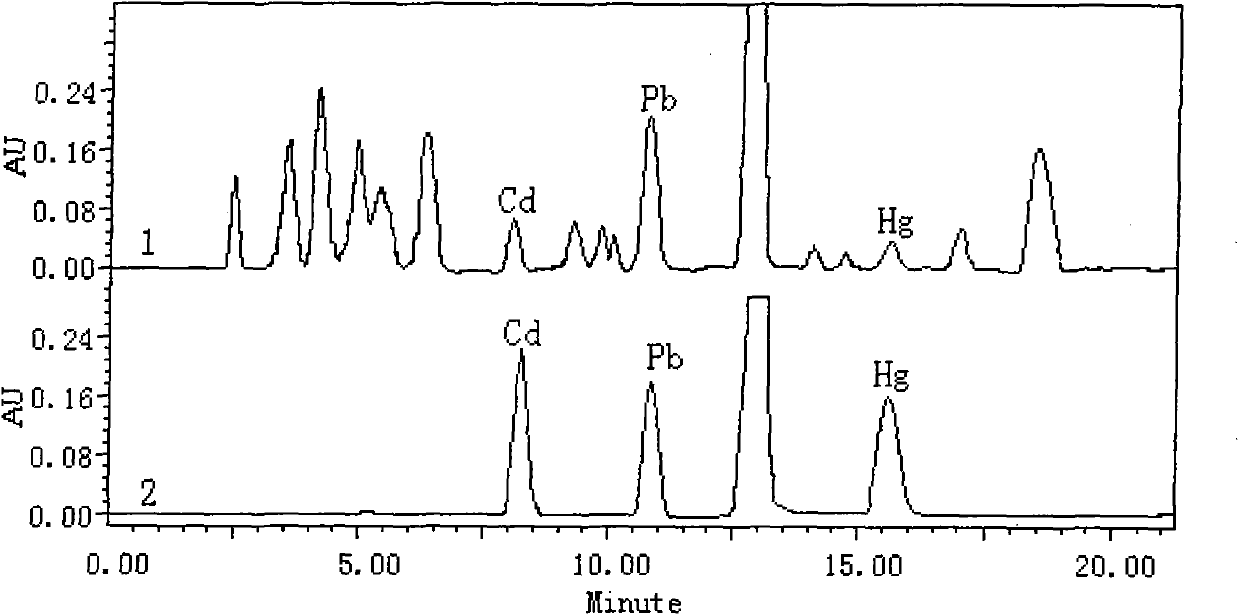
[0038] The column is Waters Xterra TM RP 18 (5μm, 3.9×150), the mobile phase is: A 0.05moL / L pH=10.0 tetrahydropyrrole-acetic acid buffer solution, B acetonitrile (containing 0.05moL / LpH=10.0 tetrahydropyrrole-acetic acid buffer solution) and The volume ratio is changed according to: 0min (A 70%+B 30%), 10min (A 40%+B 60%) linearly increasing gradient, the injection volume is 20μL, under this condition, the standard sample and the actual sample are at a wavelength of 510nm , see chromatographic comparison figure 1 .
Embodiment 3
[0040] ——Determination of lead, cadmium and mercury in tobacco
[0041] Accurately weigh 0.5 g of the tobacco sample into a polytetrafluoroethylene microwave digestion tank, add 5 mL of concentrated nitric acid and 8 mL of 30% H 2 o 2 , digested in a microwave digestion furnace for 20min; after digestion, heated and evaporated on a hot plate until nearly dry, dissolved the residue with 20mL of 5% nitric acid, and transferred it to a 50mL volumetric flask. Add 5 mL of 0.1% 5-phenylazo-8-(2,4-dihydroxybenzaldehyde)-aminoquinaridine ethanol solution to the sample digestion solution, adjust the pH value to nearly neutral, and then add pH=10.0 Dilute 10 mL of borax-sodium hydroxide buffer solution with water to near the mark, and dilute to 50 mL. The solution is passed through the activated Waters Xterra at a flow rate of 10ml / min TM RP 18 After enrichment, the solid-phase extraction column was eluted with 1.0mL ethanol at a flow rate of 10ml / min. The volume of the eluent was a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molar absorption coefficient | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com