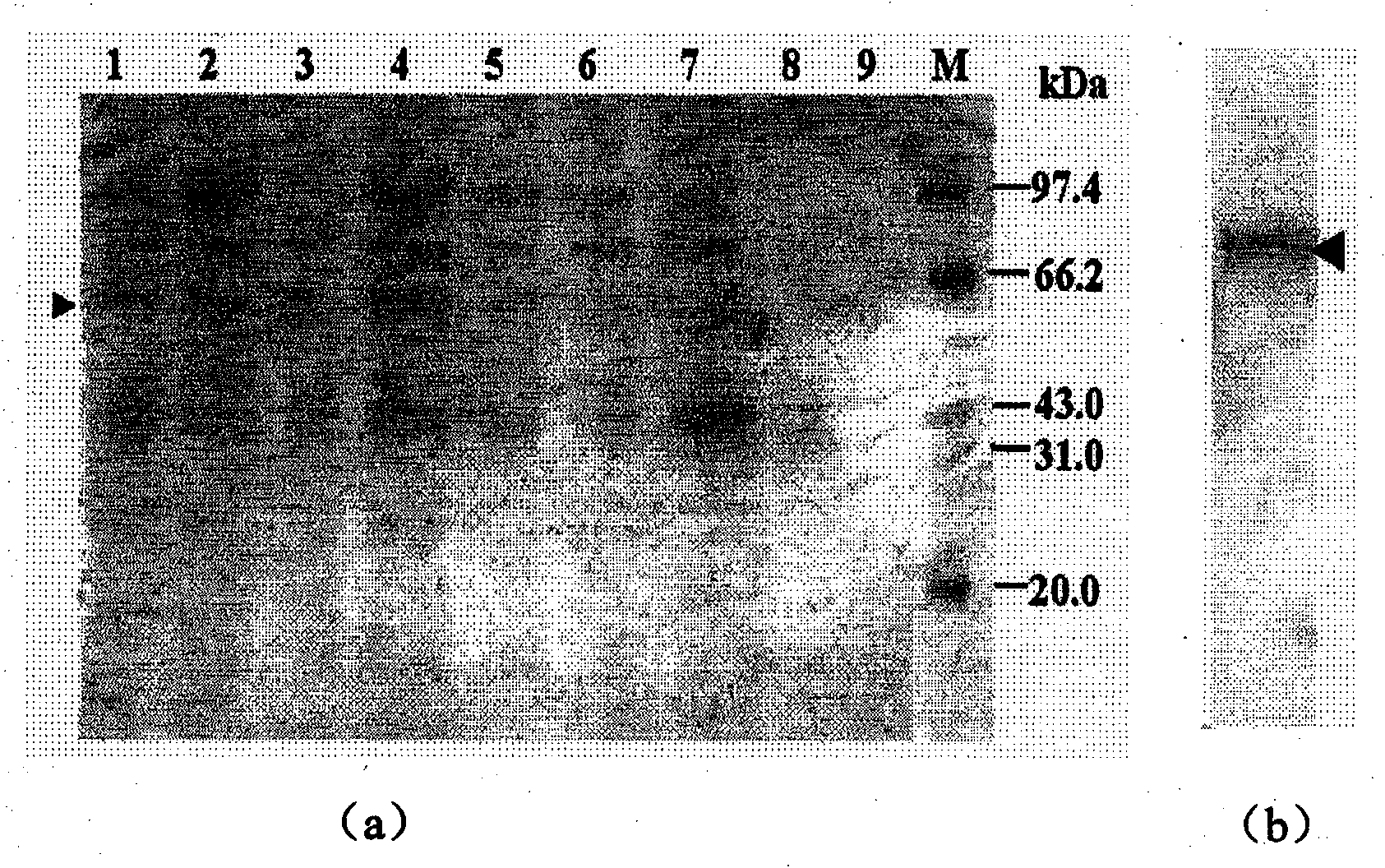
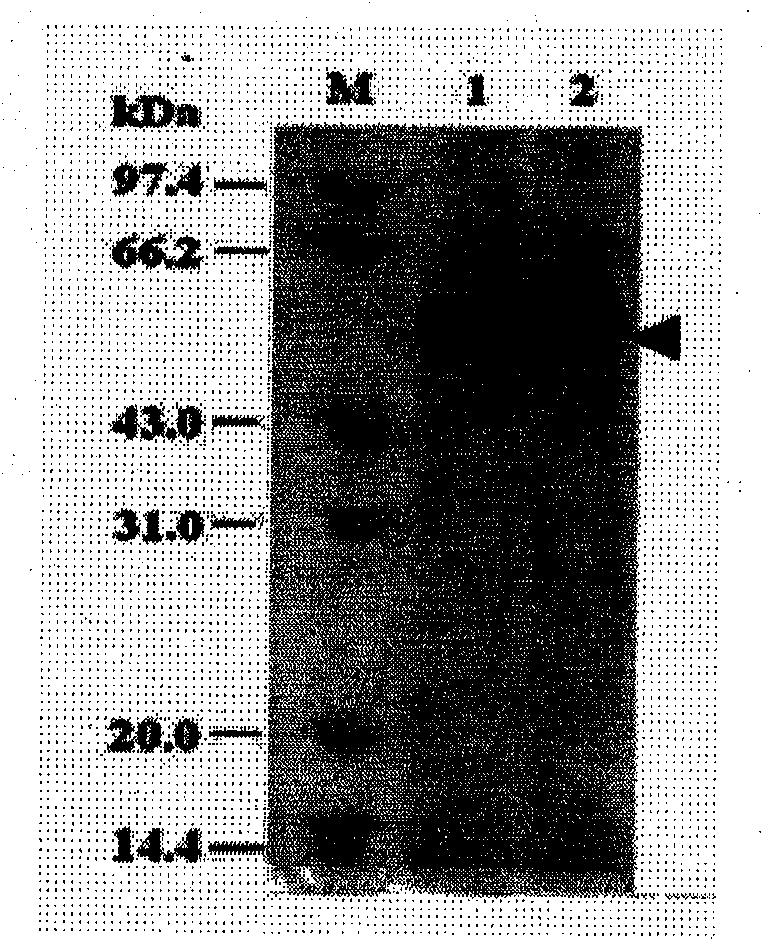
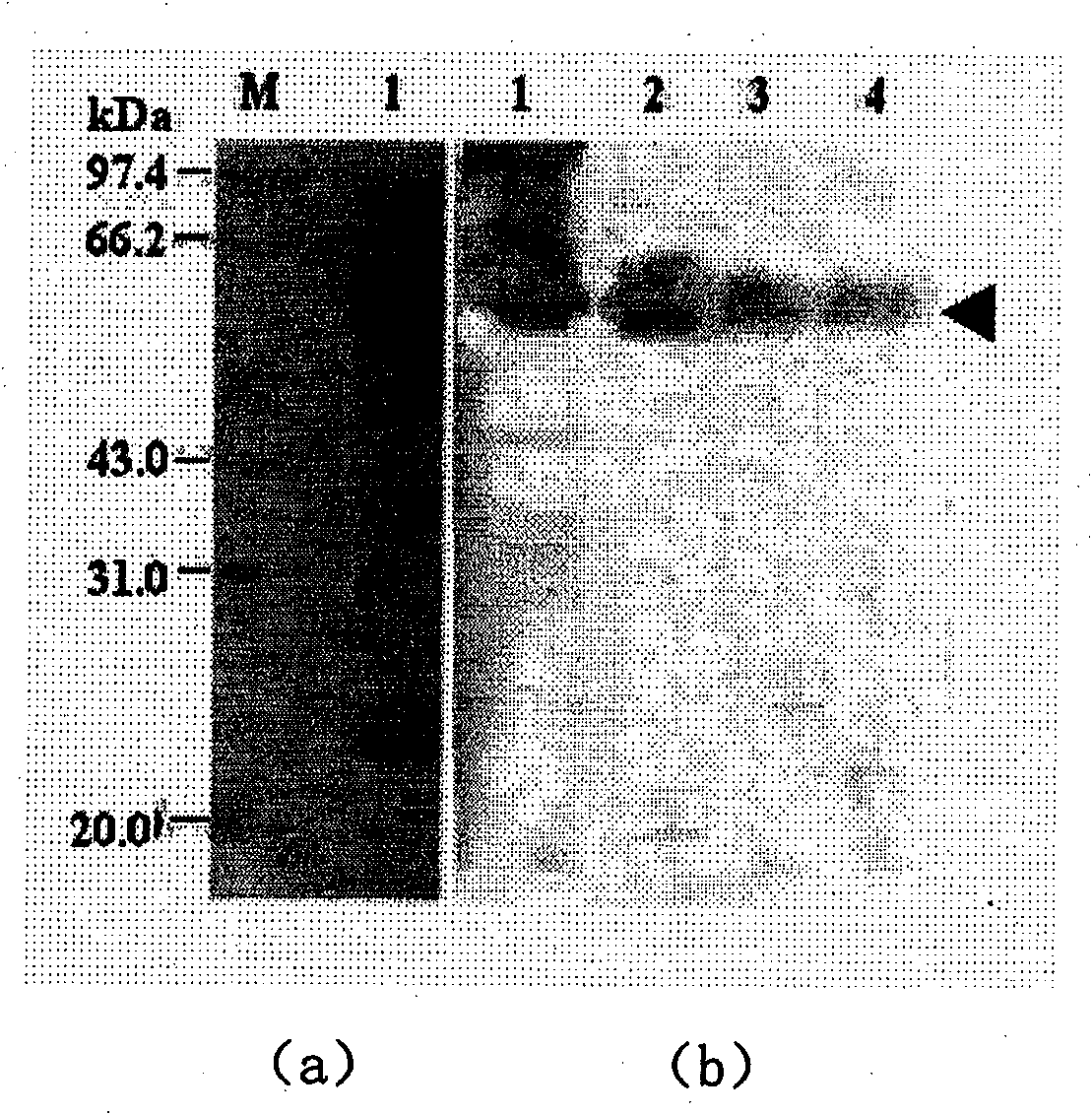
Yeast expression method for recombining major protein AccMRJP1 of apis cerana royal jelly and product application
A technology of royal jelly major protein and Apis cerana, which is applied in the field of yeast expression of recombinant royal jelly protein AccMRJP1 of Apis cerana to achieve a wide range of effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment i
[0044] Cloning of embodiment i AccMRJ1 gene
[0045] Material
[0046] All primers were synthesized by Shanghai Sangon Company:
[0047] Example of upstream primer 19k-ecor-6hisek-F: 5′-A CATCATCATCATCATCAT GCATTCTTCGAGGA-3' (sequence shown in SEQ ID NO.1), in which the underlined sequence encodes 6 histidines (6×His), which is convenient for affinity separation of expression products; the framed sequence is the enzyme cleavage site EcoR I, italics is the thrombin cleavage site, which is convenient for cutting 6 histidines from the expression product; A before the cleavage site is the protective base, and the rest is the coding sequence at the 5' end of the mature peptide of AccMRJP1.
example 1
[0048] Downstream Primer Example 1 MRJP-NOT-R: 5’-CCG TTACAGATGTATTGAAATTTTG-3' (sequence shown in SEQ ID NO.2). The framed sequence is the restriction site NotI, the CCG before the restriction site is the protective base, and the rest are complementary sequences at the 3' end.
[0049] The a-factor primer is 5'-TACTATTGCCAGCATTGCTGC-3' (sequence shown in SEQ ID NO.3), designed according to the a-factor at the 5' end of pPIC9K.
[0050] Example of upstream primer 25'AOX1: 5'-GACTGGTTCCAATTGACAAGC-3' (sequence shown in SEQ ID NO.4), designed according to the 5'-promoter sequence of vector pPIC9K, used for PCR detection of recombinant yeast vector.
[0051] Downstream primer example 2AOX1: 5'-GCAAATGGCATTCTGACATCC-3' (sequence shown in SEQ ID NO.5), designed according to the 3'-promoter sequence of vector pPIC9K, used for PCR detection of recombinant yeast.
[0052]Escherichia coli (Escherichia coli) strains TG1 and DH5α are preserved by our laboratory;
[0053] Pichia pasto...
Embodiment 2
[0062] Example 2 Construction and induced expression of highly expressed yeast recombinants
[0063] Preparation of Competent Cells
[0064] ①Stretch culture of GS115 strain on YPD solid medium (YPD liquid medium formula: peptone 20g, yeast extract 10g, glucose 20g, add deionized water 1000mL. YPD solid medium: YPD liquid medium + 1.5 agar powder) , cultured at 37°C for 1-2 days, picked a single colony of yeast, inoculated into a 50mL Erlenmeyer flask containing 5ml of YPD medium, and cultured overnight at 30°C for 250-300min. ②The next day, inoculate 100-500 μl of the culture into a 2L Erlenmeyer shaker flask containing 500 mL of fresh YPD medium, and culture overnight at 28-30°C, 250-300 rpm, until OD 600 The value reaches 1.3-1.5. ③ Centrifuge the cell culture at 4°C and 1500g for 5 minutes, and resuspend the bacterial cell pellet with 500 mL of ice-cold sterile water; ④ Centrifuge according to step 3, and use 250 mL of ice-cold sterile water to pellet the bacterial cell ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com