Polymer intensifier in controlled release preparation
A technology of controlled-release preparations and polymers, applied in the field of zero-order release controlled-release preparations and their preparation, and in the field of zero-order release controlled-release preparations, which can solve the problem of rupture of the controlled-release coating, small changes, and no change in drug content, etc. question
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0154] Each basic step in the preparation method of the controlled-release preparation is described in detail below.
[0155] 1), preparing a core material containing at least one biologically active substance
[0156] The preparation method of the core material used in the present invention is not particularly limited in the present invention. In general, core preparation methods can be used by direct extrusion methods, extrusion methods of dry, wet or sintered granules, extrusion and subsequent rounding methods, wet or dry granulation or direct pelletization (for example on discs) , or by bonding the powder (powder layer) to active substance-free spheres (granules) or active substance-containing granules, or by forming tablets in a certain way, such as by compression, or a combination of the above methods.
[0157] 2) Coating step: use a solution or aqueous dispersion of a polymer that is insoluble or almost insoluble in water and digestive juices containing sublimable subs...
Embodiment 1
[0184] Embodiment 1 and comparative example 1
[0185] 1, preparation embodiment 1 sample
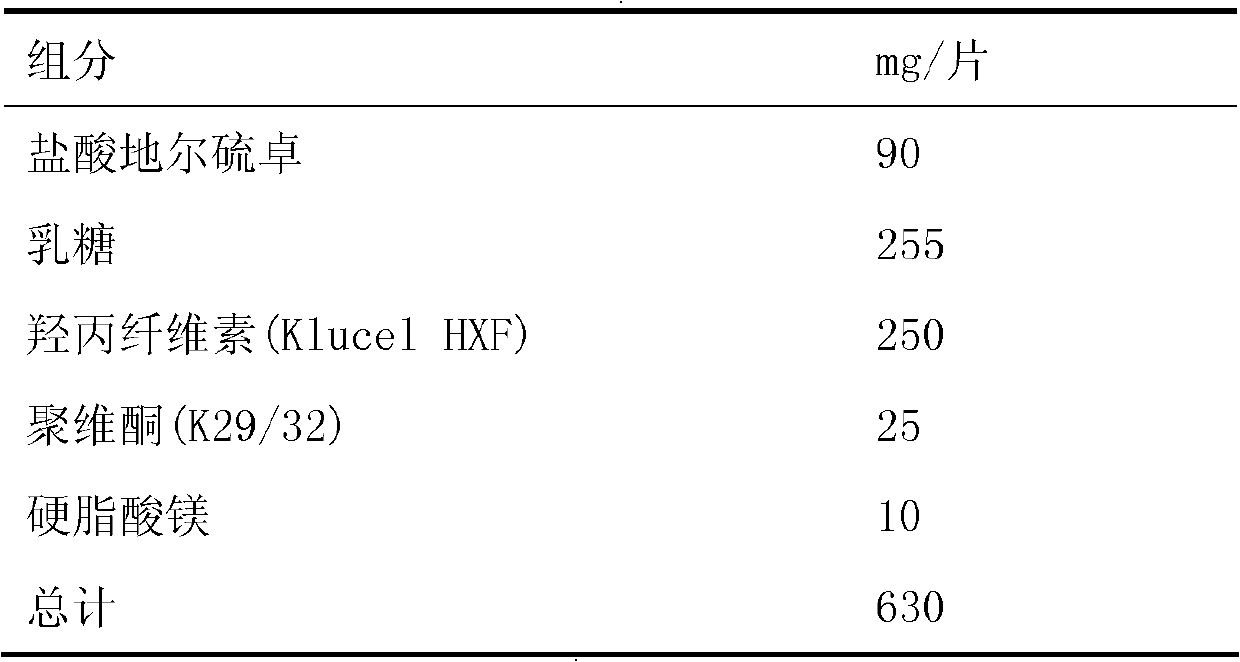
[0186] 1), prepare tablet core according to the following prescription and process:
[0187]
[0188] Mix diltiazem hydrochloride, lactose, hydroxypropyl cellulose and povidone evenly, and granulate with ethanol; force the wet granulated material to pass through an 18-mesh sieve and dry for 24 hours; after sizing, add magnesium stearate and mix Evenly, use a 12mm standard concave circular die to compress the tablet, the used compression force is 1200-2000kg, and the compression time is 2s. The hardness is 6-10kg.
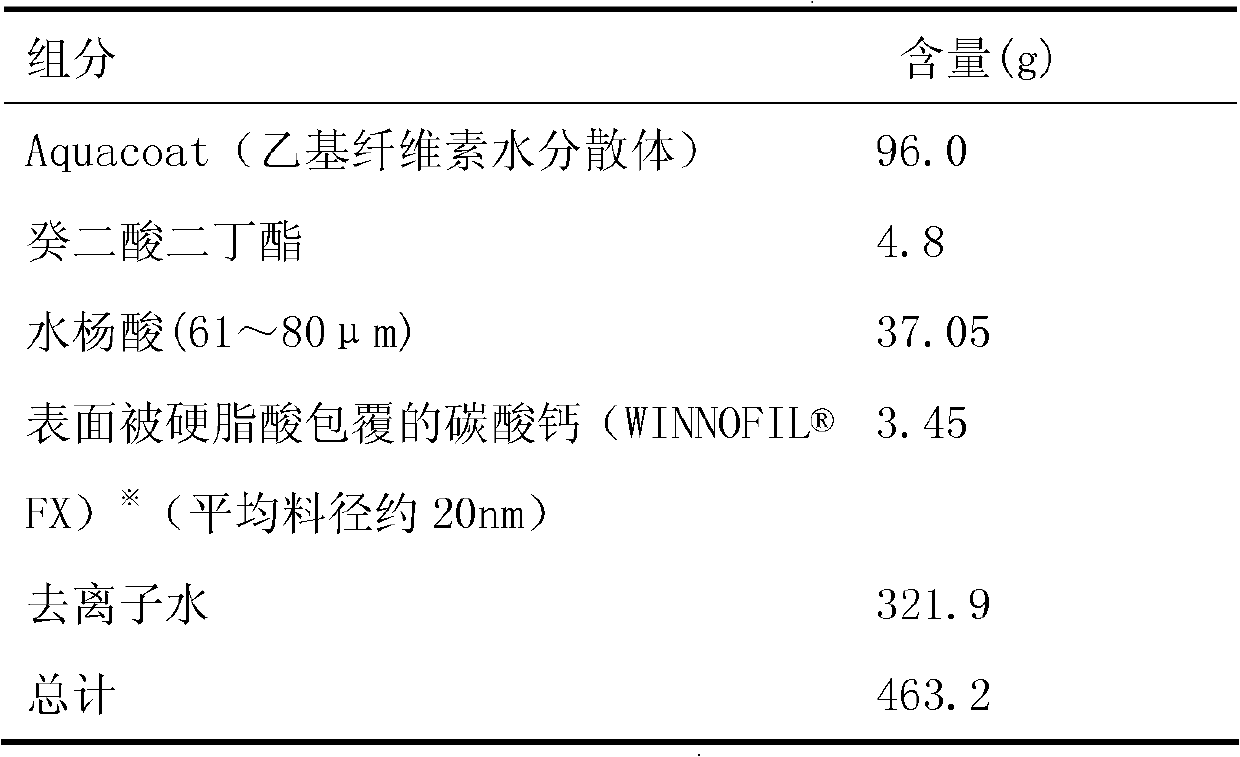
[0189] 2), the tablet core is coated according to the following prescription and process:
[0190] Coating Solution Prescription:
[0191]
[0192] ※, calcium carbonate coated with stearic acid ( FX), produced by Solvay Company, the contact angle θ with the coating polymer was measured to be 21°.
[0193] The tablet cores were coated on a Hicoater / Fruend coater. ...
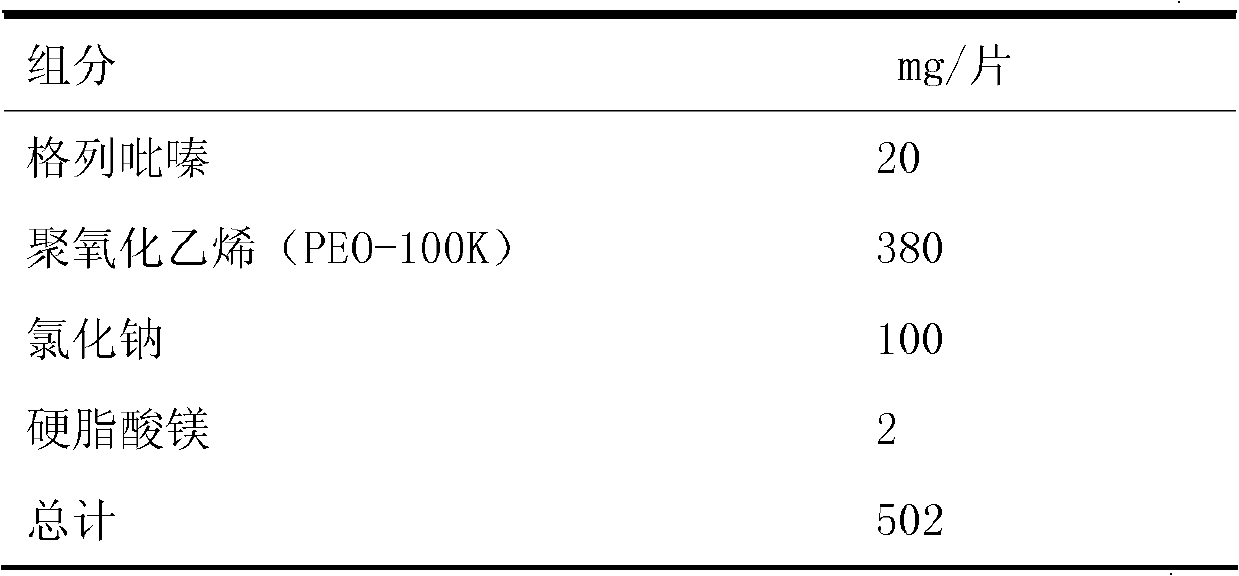
Embodiment 2
[0201] Embodiment 2 and comparative example 2
PUM
| Property | Measurement | Unit |
|---|---|---|
| Glass transition temperature | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com