PTP1B (Protein Tyrosine Phosphatase 1B) inhibitor as well as chemical synthesis method and application thereof
A chemical synthesis and inhibitor technology, applied in the field of biomedicine, can solve the problems of poor selection specificity, not much, poor chemical and biological stability, etc., and achieve the effects of low-cost synthesis, simple process operation, and good industrial production prospects.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
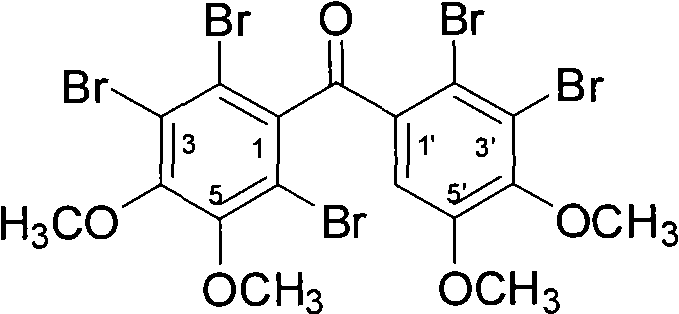
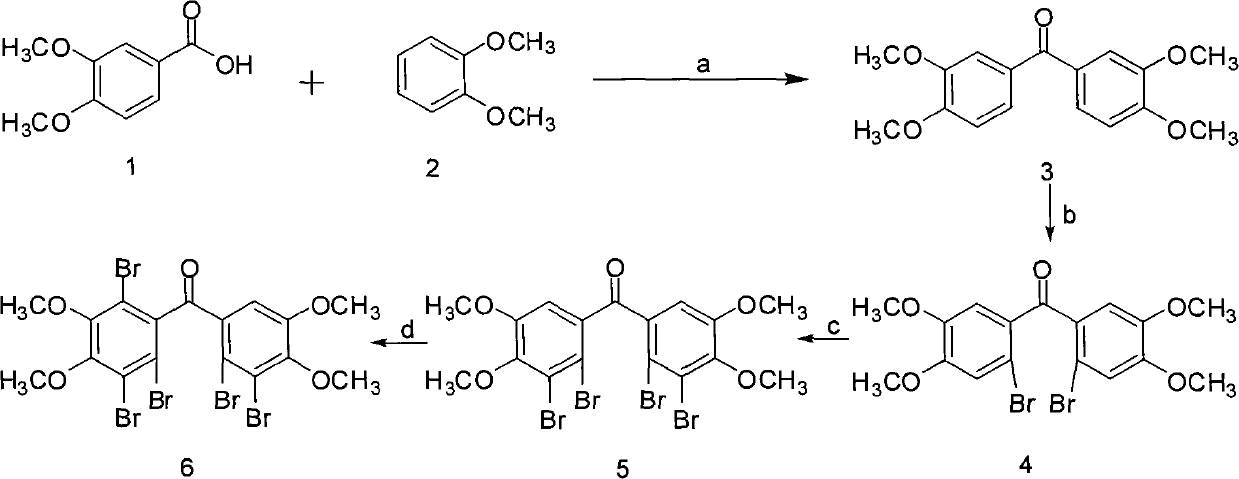
[0030] Example 1 "(2', 3'-dibromo-4', 5'-dimethoxy-phenyl)-(2,3,6-tribromo-4,5-dimethoxy-phenyl )-Methanone"Chemical Total Synthesis and Structure Identification
[0031] (1) Chemical synthesis and structure identification of Bis-(3,4-dimethoxy-phenyl)-methanone 3
[0032] Add 22.08g (120mmol) veratrol (compound 1), 16.92g (120mmol) veratrol (compound 2) and 100g polyphosphoric acid into a 250ml three-necked reaction flask according to the molar ratio of 1:1, and stir at 80°C for reaction 1h; cool to 60°C and add 250ml of ice water dropwise to the reactant within 30min, at this time, a large amount of water-insoluble pink solid precipitates out of the reactant; after removing water by filtration, dissolve the obtained solid in 100ml of dichloromethane , washed three times with an equal volume of 3% sodium hydroxide solution and distilled water successively; after drying the dichloromethane phase with anhydrous sodium sulfate, concentrate under reduced pressure to obtain a pin...
Embodiment 2
[0040] Example 2 Determination of protein tyrosine phospholipase 1B inhibitory activity
[0041] Compounds 3, 4, 5 and 6 to be tested were prepared into different concentrations of the test solution with DMSO, and 2 μL of the test solution was added to the standard bioassay system (50mM Tris-HCl, pH 6.5, 2mM pNPP , 2% DMSO, 30nM hGST-PTP1B), negative control: DMSO, positive control: sodium orthovanadate, the reaction temperature is 30°C, the dynamic measurement wavelength is the light absorption at 405nm, the time is 3min, and the compound PTP1B enzyme is calculated according to the following formula Vitality inhibition rate. Inhibition rate=(experimental group A value-negative control group A value) / (control group A value-negative control group A)×100%, the results are shown in Table 1.
[0042] Table 1 Inhibition rate of protein tyrosine phospholipase 1B (%)
[0043] Table 1 Inhibitory Ratio(%) of PTP1B
[0044]
[0045] When the compound concentration is 20 μg...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com