Technetium-labeled estradiol derivative and reference compound thereof, and preparation method, application and intermediate thereof
A compound and estradiol technology, applied in the field of technetium-labeled estradiol derivatives and reference compounds, can solve the problems of poor imaging effect, poor target tissue selectivity, and high lipophilicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
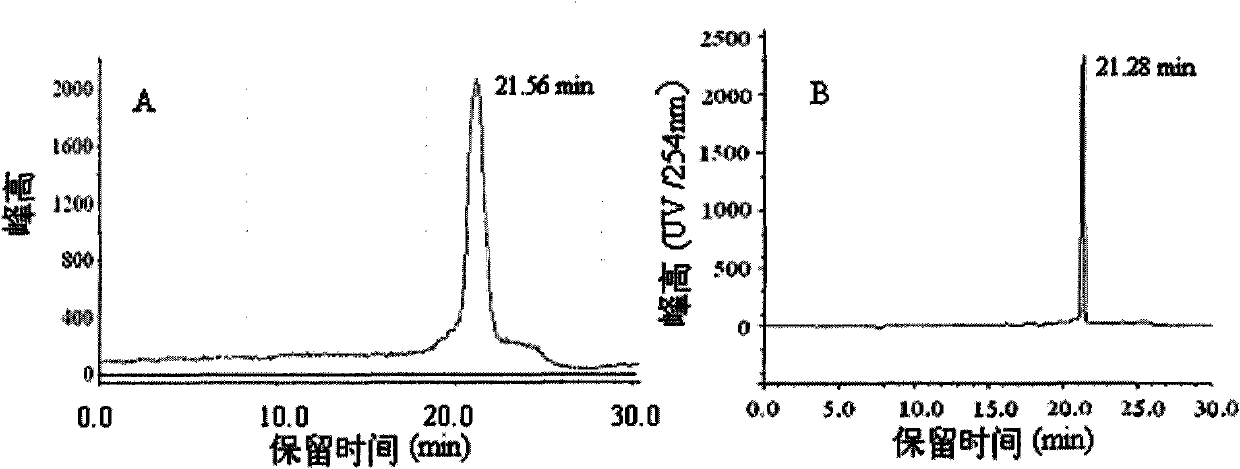
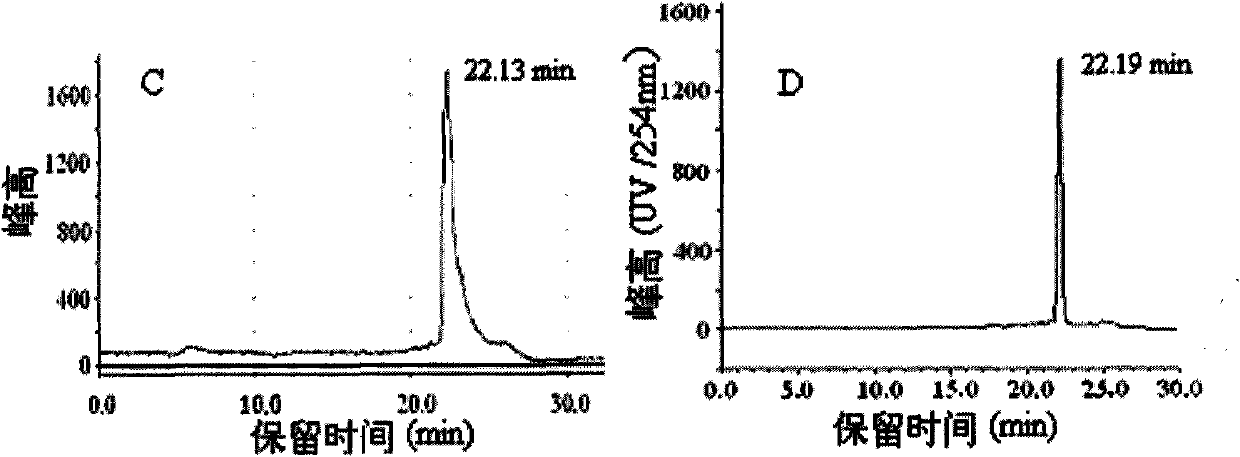
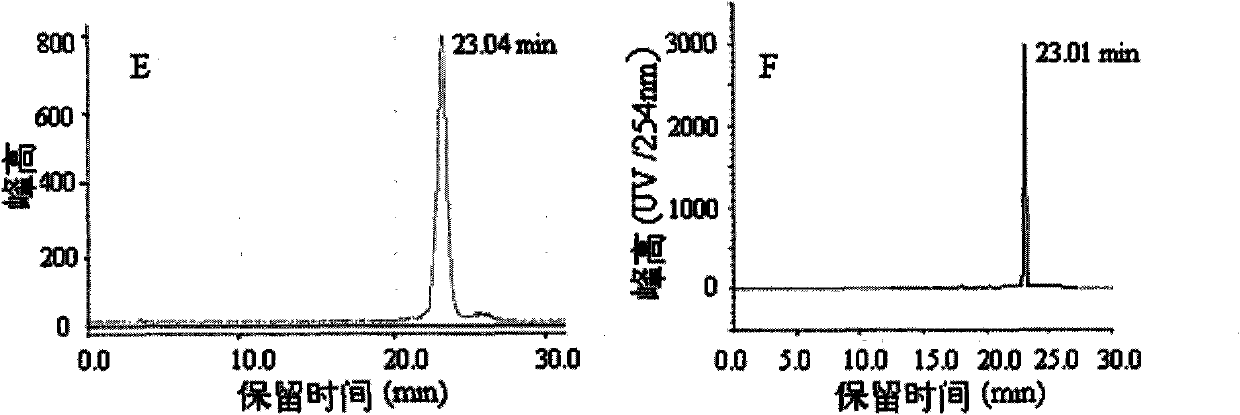
Image
Examples
preparation example Construction
[0091] (1) The specific steps of the preparation method are as follows: after dissolving 5g (18.7mmol) of estrone in 25ml of methylene chloride, add 0.8g (3.2mmol) of pyridinium p-toluenesulfonate under magnetic stirring, and then add 4.8ml ( 46.6mmol) of 3,4-dihydropyran was reacted at 22°C for 20 hours. After the reaction, an appropriate amount of distilled water was added and extracted with dichloromethane (3×20ml). The organic phase was respectively washed with saturated sodium bicarbonate solution, water and saturated brine, and then dried with anhydrous sodium sulfate. After evaporating the solvent under reduced pressure, several white solids were obtained with a yield of 99% and a melting point m.p. of 137.1-139.2°C.
[0092] The identification data of compound I are as follows:
[0093] IR(KBr): v(=C-H)3019, v(C-H)2978, v(C=O)1736cm -1 .
[0094] 1 HNMR (CDCl 3 ): δ0.90(s, 3H, 18-CH 3 ), 1.41~1.71(m, 9H), 1.82~1.85(m, 2H), 1.94~2.07(m, 4H), 2.09~2.17(m, 1H), 2.23~...
Embodiment 2
[0097] Embodiment 2 Synthesis of formula II compound 3-(tetrahydropyran-2-methyleneoxy group)-16β-methoxycarbonyl estrogen-1,3,5(10)-triene-17-ketone
[0098]
[0099] (1) The specific steps of the preparation method are as follows: add 40ml of anhydrous tetrahydrofuran into a double-neck flask, then add 7.5g (56.3mmol) of potassium hydride that is dispersed in the oil with a mass fraction of 30% under the condition of nitrogen protection. After stirring for 10 minutes, add 4ml (47.5mmol) of dimethyl carbonate, then add 6.6g (18.8mmol) of compound I dissolved in 50ml of tetrahydrofuran, under nitrogen protection conditions, heat to reflux for 2.5 hours, cool to room temperature, dropwise Add water to remove unreacted potassium hydride, then extract with ethyl acetate, wash the organic phase once with saturated ammonium chloride, water and saturated brine, dry over anhydrous sodium sulfate, flash column chromatography (eluent: petroleum ether / The volume ratio of ethyl aceta...
Embodiment 3
[0105] Example 3 Compound III a~b Synthesis
[0106]
[0107] (1) Compound III a : 16α-butyl bromide-16β-methoxycarbonyl-3-(tetrahydropyran-2-methyleneoxy)estrogen-1,3,5(10)-trien-17-one synthesis
[0108] Among them, A is the group -(CH 2 CH 2 ) x -, x is 2; R is bromine.
[0109] The specific steps of the preparation method are as follows: dissolve 0.5g (1.22mmol) of compound II in 10mL of dichloromethane, then add 0.1g of benzyltriethylammonium chloride, 1.05g of 1,4-dibromobutane and 5ml of 10% NaOH solution (w / v), react under reflux conditions for 16h, spin off the solvent under reduced pressure, add water, extract with ethyl acetate, wash the organic phase with water and saturated brine once, dry over anhydrous sodium sulfate, filter, evaporate under reduced pressure The solvent was removed and flash column chromatography (volume ratio of eluent: petroleum ether / ethyl acetate: 20:1) gave a colorless oily liquid with a yield of 79%.
[0110] Compound III a The...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com