Qingpeng emplastrum for reducing swelling and stopping pain and preparation method thereof
A plaster, swelling and pain-relieving technology, which is applied in the directions of non-central analgesics, medical preparations with inactive ingredients, and medical preparations containing active ingredients, etc. It can reduce the dosage of drugs, improve the bioavailability, and the effect of long duration of action.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0114] According to the specific embodiment of the present invention, the preparation method of Qingpeng plaster of the present invention can comprise a kind of in following A, B and C method:
[0115] A method:
[0116] ① Grind musk into fine powder;
[0117] ②Sort Oxytropis chinensis, subrhubarb, iron rod hammer, pitted myrobalan, myrobalan, emblical, benzoin, and vine, and crush them into medicinal powder with a particle size of 0.1-150 μm;
[0118] ③ Fully mix the above-mentioned medicinal powder with the above-mentioned musk fine powder, water-soluble polymer, transdermal accelerator, humectant, cross-linking agent, pH regulator, antibacterial agent and water, stir evenly, apply, condense, and cover the lining film , cutting, and packing to get Qingpeng plaster of the present invention.
[0119] Method B:
[0120] ① Remove impurities from Oxytropis chinensis, subrhubarb, iron rod hammer, seed myrobalan, myrobalan myrobalan, emblica, benzoin, vine vine, clean and mix; ...
experiment example 1
[0267] Experimental example 1: Screening experiment of auxiliary materials of Qingpeng plaster of the present invention
[0268] 1. Skeleton adhesive screening
[0269] The Chinese Pharmacopoeia 2010 Edition, an appendix "Plasters", stipulates under the General Rules of Plaster Preparations that the dosage form "commonly used bases include natural rubber, thermoplastic rubber, rosin, rosin derivatives, vaseline, lanolin and zinc oxide. It can also be used Other suitable solvents and matrices".
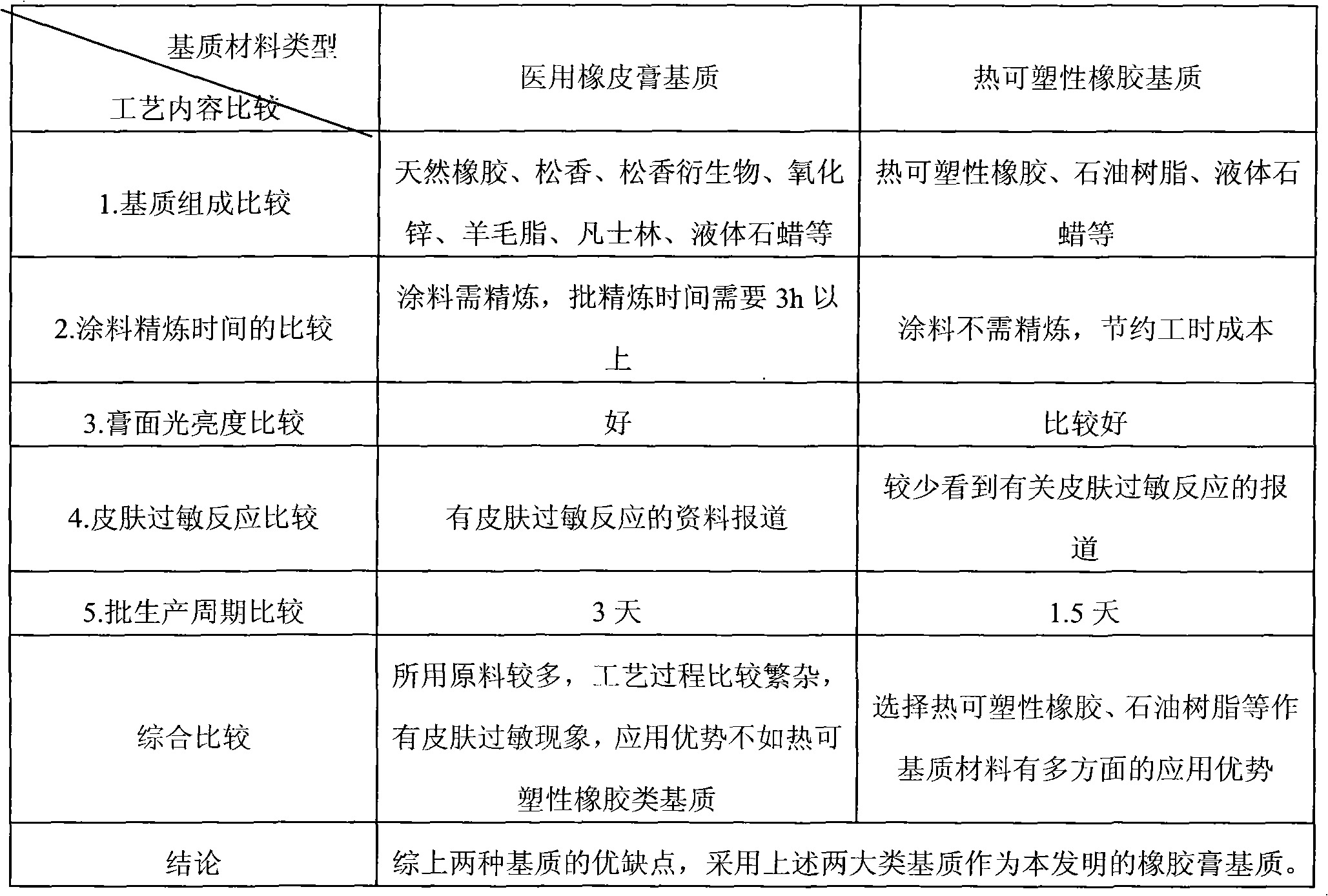
[0270] During the research process of the preparation process, the two major types of bases for the production of plasters were analyzed emphatically, and their respective advantages and disadvantages were screened and compared. The results are shown in Table 1.
[0271] Table 1 Comparison screening table of advantages and disadvantages of coatings prepared from two different matrix materials
[0272]
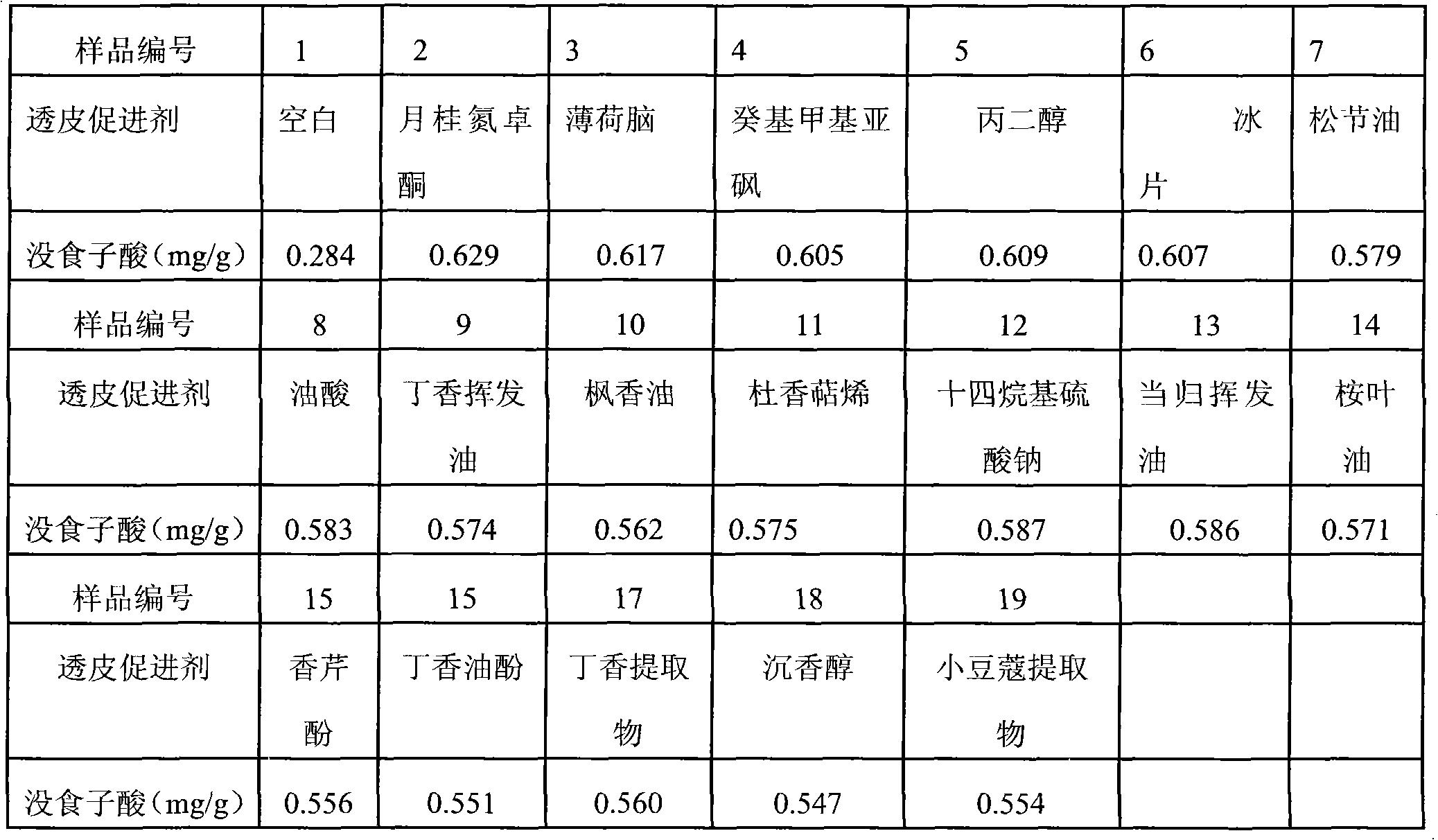
[0273] 2. Screening experiment of skin penetration enhancer:
[0274] ① Selec...
experiment example 2
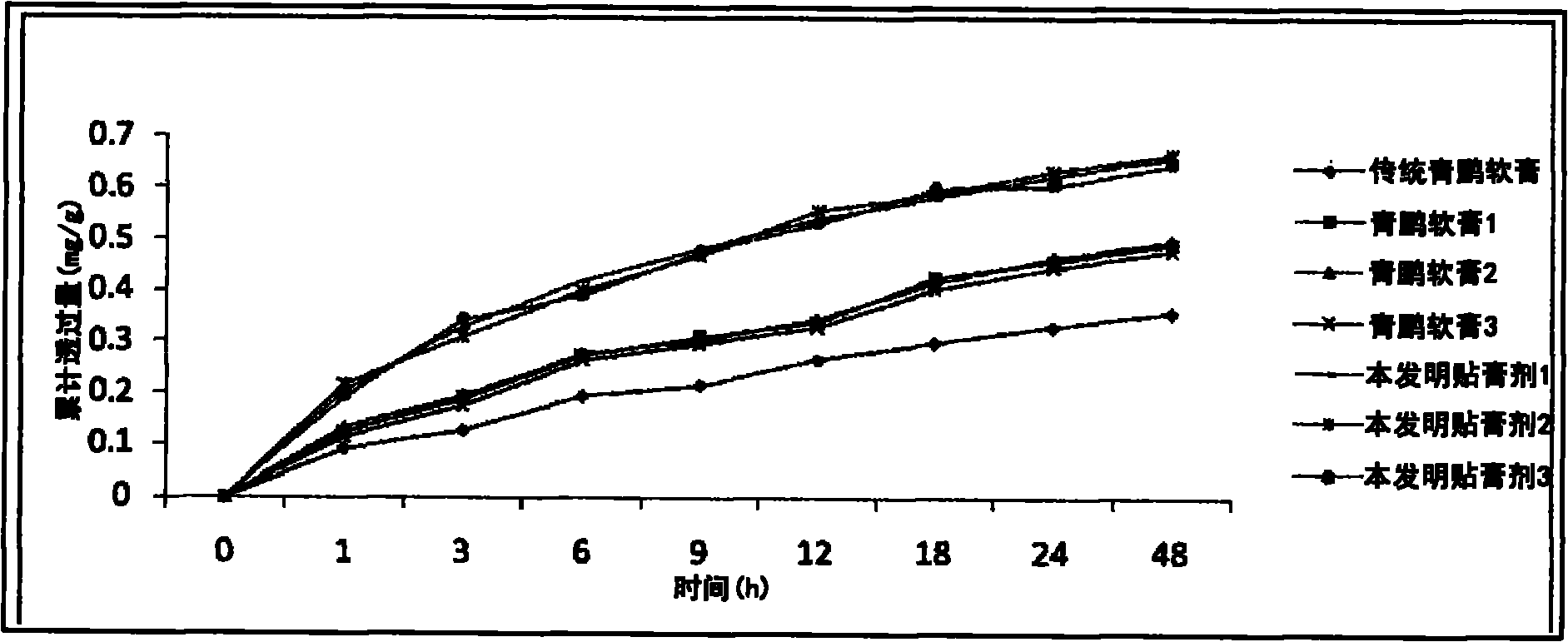
[0315] Experimental Example 2: Comparative Experimental Data of Qingpeng Paste Plaster of the Present Invention and Qingpeng Ointment
[0316] Experimental sample:
[0317] Traditional Qingpeng ointment: provided by Tibet Qizheng Tibetan Medicine Co., Ltd.;
[0318] Qingpeng Ointment 1: Preparation method: ① Purify Oxytropis 100g, Subrhubarb 50g, Iron Bar Hammer 75g, Nucleus myrobalan 100g, Fructus myrobalan 100g, Emlical emblica 100g, benzoin 35g, and vine vine 150g, crush them , mixed evenly, and set aside; ②Weigh 50% of the total weight of the above-mentioned mixed raw materials, use 2.13kg of water, decoct 3 times at 85°C, and decoct for 2 hours each time, collect the extract and volatile oil respectively; filter, Concentrating the extract to a concentrated solution with a relative density of 1.0 to 1.5; adding the volatile oil to the concentrated solution; ③ pulverizing the above-mentioned remaining mixed raw medicine into a 100 μm powder, and mixing it with the above-me...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com